The prevalence of combination vaccines for children in Europe. Analysis of the availability and funding
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
The article presents an analysis of data from 33 European countries on combination vaccines against diphtheria (D), tetanus (T), pertussis (P), polio (IPV), Haemophilus influenzae type b (Hib), hepatitis B (HBV). The purpose of this article is to present the availability and funding mechanisms of vaccinations which represent pharmacological innovations.
The performed analysis included 5 approved combination vaccines marketed in Europe (5-in-1 DTaP-IPV-Hib vaccines, and 6-in-1 DTaP-IPV-Hib-HepB vaccines). Although in the vast majority of countries the analysed vaccinations are not mandatory, the vaccination rate remains very high. Significant differences between the European countries can be seen in vaccination schedules or the types of available combination vaccines in the national childhood immunisation programmes. Despite the different rules and regulations regarding the funding of combination vaccinations from public resources, countries systematically seek to provide patients with the latest generation formulations.
Introduction
Combination vaccines representing pharmacological innovations are not commonly used. Combination vaccines are vaccines comprising two or more microbial agents or their antigens combined in a single dose. They are administered at the same time at the same anatomical site and provide combined immunity against at least two diseases. Combination vaccines reduce the need for multiple injections, which are necessary to prevent infectious diseases.
In Poland and in the European Union/European Economic Area (EU/EEA) countries, 5 in 1 against diphtheria, tetanus, pertussis, polio and Hib vaccines (Infanrix IPV + Hib, Pentaxim) and 6 in 1 vaccines against diphtheria, tetanus, pertussis, polio, Hib and hepatitis B (Infanrix hexa, Hexacima) are available. These diseases have been largely eliminated or reduced owing to actions taken by the European countries in providing access to vaccinations. This article aims to analyse the differences in the planning, organisation and funding of combination vaccines for the population of children in Europe.
Methods
In this article we present information that allowed us to compare the availability, funding mechanisms, and how and where combination vaccines are purchased in the European countries. The information collected was based primarily on data collected from experts in each country.
The analysis of the solutions adopted in the field of immunisation against diphtheria (D), tetanus (T), pertussis (P), polio (IPV), Haemophilus influenzae type b (Hib), hepatitis B (HBV) vaccines was performed for both pentavalent and hexavalent vaccines in the paediatric population. Analysis of the existing legal regulations, organisation and funding mechanisms for vaccinations covered 331 countries in Europe.
countries in Europe.
Data on the epidemiology of vaccination published by the European Centre for Disease Prevention and Control (ECDC) and Eurostat were used in the analysis. The publicly available information was supplemented by collecting additional data directly from the analysed countries. The recommendations and guidelines for vaccination are from the Web sites of the Centre for Disease Control and Prevention (CDC) or the European Office of the CDC (ECDC), and the World Health Organization (WHO).
Epidemiology
In 2010, 14 cases of diphtheria were reported in the EU countries; the incidence rate was below 0.01/100,000 for the European population. The majority of cases were women over 45 years of age. Diphtheria has been almost completely eliminated in Europe; however, it is still present in the former Soviet Union countries (especially in Ukraine and Russia), and therefore there are cases of sporadic epidemics in the world, especially when the vaccination rate is insufficient. This situation points to the need for vaccination in all age groups [1].
Tetanus is a very rare disease in the EU/EEA countries, caused by the bacterium Clostridium tetani, mainly due to effective vaccination programmes in all countries and generally good standards of hygiene. The incidence rate is very low at 0.02/100,000 for the European population. Vaccinations against tetanus are included in the immunisation schedules of all EU countries for the youngest age groups; however, it is suggested that there is a need for additional vaccination in adults, especially those who have not been vaccinated in their childhood [1].
In 2010, 13,964 confirmed cases of pertussis have been reported in the 28 EU/EEA countries2 giving an average incidence rate of 3.87/100,000. Data from Germany and Liechtenstein are not shown in the cited analysis. However, this rate varies between countries, being the highest in Estonia (95.55/10,000) and the lowest in Spain (0.66/100,000). Most cases involved groups of children aged 5 to 14 years, and adolescents. Consequently, countries such as: Austria, Belgium, France, Finland, Germany, Norway and Italy have introduced additional vaccinations in adolescents in their immunisation programmes. It should be noted that pertussis is often misdiagnosed in adolescents and adults, and as a result, they can infect younger children [1].
giving an average incidence rate of 3.87/100,000. Data from Germany and Liechtenstein are not shown in the cited analysis. However, this rate varies between countries, being the highest in Estonia (95.55/10,000) and the lowest in Spain (0.66/100,000). Most cases involved groups of children aged 5 to 14 years, and adolescents. Consequently, countries such as: Austria, Belgium, France, Finland, Germany, Norway and Italy have introduced additional vaccinations in adolescents in their immunisation programmes. It should be noted that pertussis is often misdiagnosed in adolescents and adults, and as a result, they can infect younger children [1].
In 2002, the World Health Organization (WHO) announced European a polio-free region. In 2010, 478 confirmed cases were reported in Tajikistan (460 cases), Turkmenistan, Russia and Kazakhstan. In most cases, the disease was caused by a wild strain of the WPV1 virus. The virus was probably brought to these countries from India. However, in spite of an epidemic outbreak in Tajikistan, there were no cases of illness caused by the WPV1 virus over the next 12 months in the European countries. There was only one case of acute epidemic post-vaccination paralysis in Poland in 2010, which has no effect on the above figures [1].
In 2010, 14,745 confirmed cases of hepatitis B have been reported in the 27 EU/EEA countries (except for Belgium, Italy and Liechtenstein, which did not provide data), giving an incidence rate of 3.43/100,000. The disease usually occurs in people aged 25 to 34 years (33.2% of all cases), more often in men (8.79/100,000 of the male population) than in women (7.42/100 000 of the female population) [1].
Haemophilic bacteria type b (Haemophilus influenzae [Hib]) is a common bacterium that causes inflammation of the respiratory tract (e.g., bronchitis, sinusitis) and otitis media, as well as more serious, life-threatening diseases: meningitis, sepsis, epiglottitis, pneumonia. In the years 1990–2009, all EU member states have implemented mandatory vaccination of young children against Hib; as a result, the incidence of this bacterial infection has become very rare. In 2010, there were only 1,970 confirmed cases of Hib infections (data from 29 countries3 ).
).
Invasive Hib infection are most common in children under 5 years of age (incidence rate 1.014/100,000), and in adults over 65 years (1.17/100,000). The incidence is dependent on the season of the year, with an increase in the winter months [1].
Guidelines and recommendations
The Advisory Committee on Immunization Practices (ACIP) guidelines for vaccination schedules against diphtheria, tetanus and pertussis indicate the need to start vaccination at 2, 4, 6 months of age, and then in months 15–18 and at 6 years of age, but not earlier than at 6 weeks of age. Children older than 7 years, which have not been previously vaccinated, should be vaccinated at the age of 11–12 years, and then every 10 years.
Hepatitis B vaccination is recommended for all children aged 0–18 years; ideally, the first dose should be given to the newborn already at the hospital, another dose at the age of 1–2 months, and the last dose at the age of 6–18 months of age. However, a 3-dose vaccination series can be started at any age, with maintaining the appropriate intervals between doses.
The polio vaccine, as recommended in the ACIP guidelines, should be given in four doses in children aged 2, 4, 6–18 months of age and between 4 and 6 years of age.
The Hib vaccine, depending on the vaccine type, is administered in 3 or 4 doses at 2, 4, 6 months (optionally), then between 12 and 15 months of age [7].
The World Health Organization (WHO) recommends vaccination of newborns for hepatitis B as early as possible. For vaccination against pertussis, diphtheria and tetanus (DTP), WHO recommends that three doses are administered in the first year of life. In areas where pertussis represents a particular risk for younger infants, DTP administration should start from 6 weeks of age with 2 consecutive doses given at an interval of 4–8 weeks.
Vaccination against Haemophilus influenzae type b should be given as soon as possible after 6 weeks of age. Three doses of the vaccine should be administered at the same time as DTP. Vaccination against Polio (IPV) should be performed three times at intervals of 4 weeks, for example in weeks 6, 10 and 14 weeks or at 2, 4, 6 months of age [8].
The availability of combination vaccines in the European market
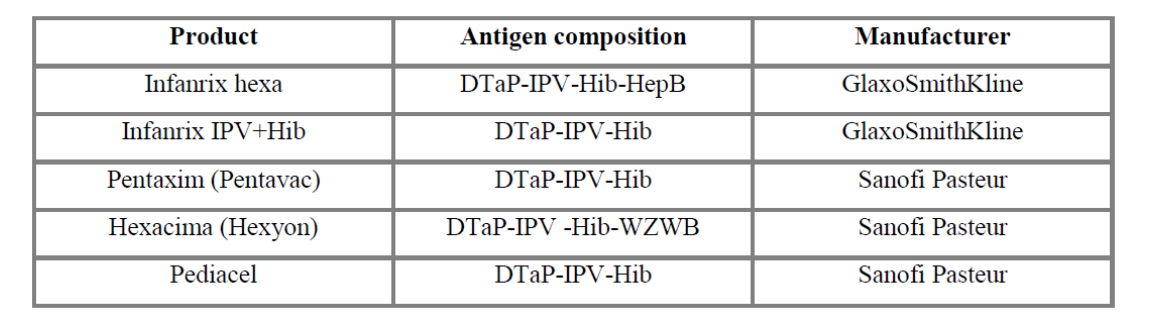
Infanrix hexa is a product in the form of powder and suspension for suspension for injection in a pre-filled syringe, used for vaccination against diphtheria (D), tetanus (T), pertussis (acellular, component) (Pa), hepatitis B (rDNA) (HBV), poliomyelitis (inactivated) (IPV) and Haemophilus influenzae type b (Hib) conjugate (adsorbed). Infanrix hexa is used for immunisation of children under 3 years. The product has been available since 2000 [9].
Infanrix IPV + Hib is a vaccine against diphtheria, tetanus, pertussis, poliomyelitis (inactivated), Haemophilus influenzae type b (conjugated, adsorbed). The vaccine is indicated for children from 2 months to 36 months of age. The vaccine has the form of powder and suspension for suspension for injection. The product was licensed in the European Union in 2000 [5].
In April 2013, the European Commission has approved another paediatric vaccine (Hexacima) for use in primary and booster vaccination of infants against pertussis, diphtheria, tetanus, hepatitis B, polio and invasive infections caused by Haemophilus influenzae type b. This vaccine, although it was approved only in the second quarter of this year, is already available in Germany and Ireland. This product is available under the trade name Hexyon in Western Europe, and under the trade name Hexacima in Eastern Europe. The product has been approved for sale in the markets across the European Union on 17 April 2013 [6].
The vaccine is in liquid form and it is used in children from six weeks to two years of age.
Pentaxim is a powder and suspension for suspension for injection. It is a vaccine against diphtheria, tetanus, pertussis, poliomyelitis (inactivated) and haemophilus type b (conjugated, adsorbed). The vaccine is used in children from 6 weeks of age. Pentaxim is also available under the name Pentavac. The first marketing authorisation was issued in 2003 [7].
Pediacel, similarly to Pentaxim, is a 5-component vaccine against diphtheria, tetanus, pertussis, poliomyelitis (inactivated) and haemophilus type b. It is available in 29 European countries4 [8] since November 2010.
[8] since November 2010.
It is worth noting that DTP vaccines have been included in the childhood immunisation programmes of the European countries for many years. The epidemiological needs necessitated the introduction of vaccines against polio, hepatitis B and infections caused by Haemophilus influenzae type b in the vaccination schedules. New technologies have made it possible to replace monovalent vaccines with tetra-, penta- or hexavalent products.
Mandatory and recommended vaccinations
In the vast majority of European countries, vaccination against pertussis, diphtheria, tetanus, poliomyelitis, Haemophilus influenzae type b infections and hepatitis B is not obligatory. The vaccinations are mandatory in nine countries, namely Bulgaria, Slovakia, Lithuania, Latvia, Poland, Croatia, Italy, Spain and Slovenia5 . In the United Kingdom and Norway, hepatitis B vaccination is recommended only for specific risk groups. However, in Norway and the United Kingdom, pentavalent vaccines are available free of charge to the entire cohort of children. In France, vaccinations are recommended and reimbursed for the entire population of children between 2 and 12 months of age, when the primary vaccination cycle and a booster dose are completed. In Serbia, combination vaccines are recommended, but they are not currently included in the national immunisation programme; it is planned to introduce them in 2014. A similar situation exists in Albania, Macedonia, Cyprus and Malta, where vaccinations for children are recommended, but they have not been included in the vaccination programme. However, in the other analysed countries6
. In the United Kingdom and Norway, hepatitis B vaccination is recommended only for specific risk groups. However, in Norway and the United Kingdom, pentavalent vaccines are available free of charge to the entire cohort of children. In France, vaccinations are recommended and reimbursed for the entire population of children between 2 and 12 months of age, when the primary vaccination cycle and a booster dose are completed. In Serbia, combination vaccines are recommended, but they are not currently included in the national immunisation programme; it is planned to introduce them in 2014. A similar situation exists in Albania, Macedonia, Cyprus and Malta, where vaccinations for children are recommended, but they have not been included in the vaccination programme. However, in the other analysed countries6 , vaccination are carried out in the entire population using combination vaccines.
, vaccination are carried out in the entire population using combination vaccines.
Availability of combination vaccines in the Universal Mass Vaccination
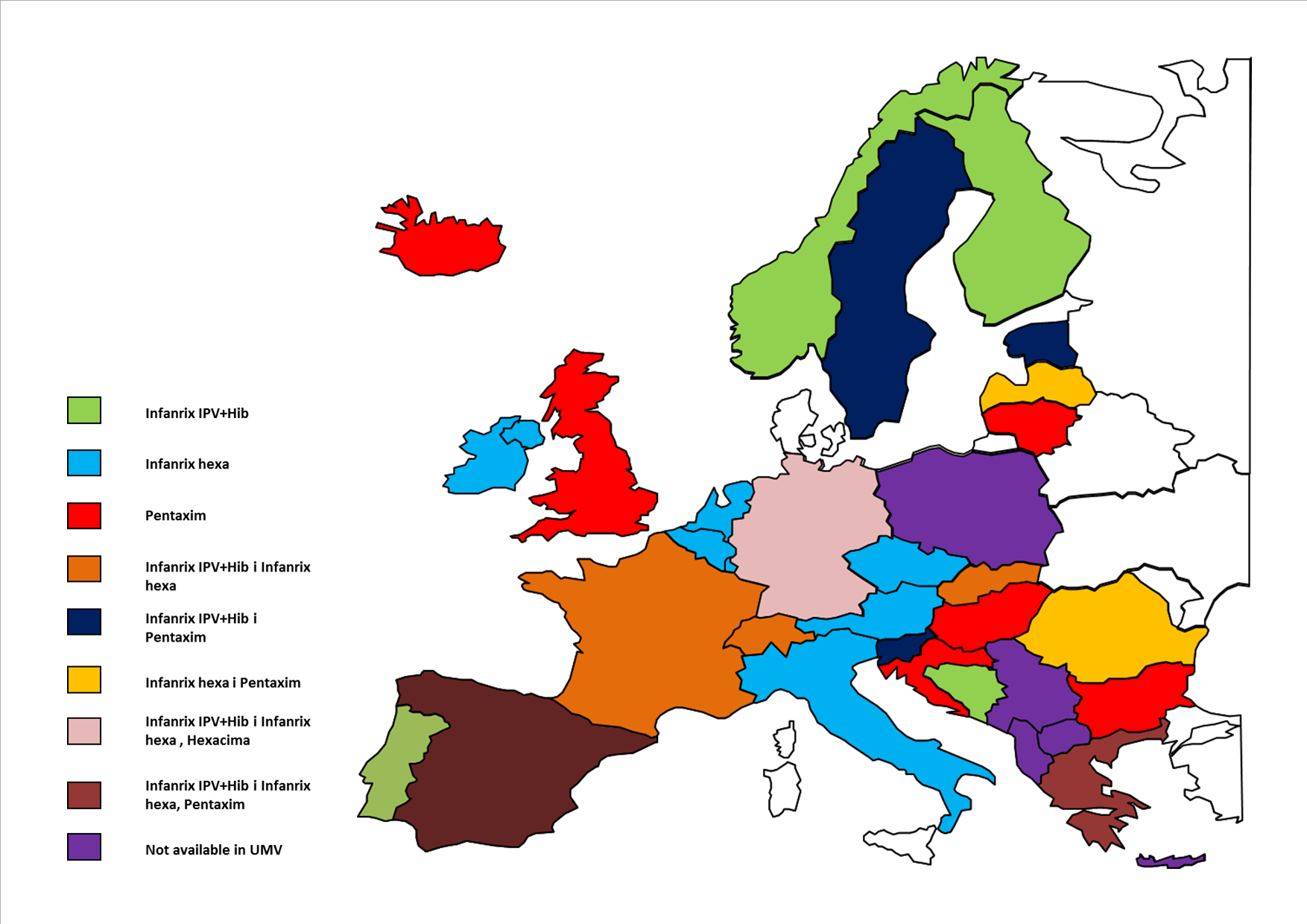
In 2013, both 5- and 6-component combination vaccines are broadly available in all 33 European countries analysed.
Various products are used as part of the national immunisation programmes in Europe against diphtheria (D), tetanus (T), pertussis (P), poliomyelitis (IPV), Haemophilus influenzae type b (Hib), hepatitis B (HBV). These differences relate to the type of product used for immunisation in children, i.e. whether it is a 6-in-1 vaccine (DTP+IPV+Hib+Hepatitis B), a 5-in-1 vaccine (DTP+IPV+Hib), or DTP, IPV and Hib administered separately. Bulgaria, Estonia, Slovenia, Sweden and Lithuania, two types of pentavalent vaccines are available in the national immunisation programmes, Infanrix IPV+Hib and Pentaxim. In Iceland, from 2013, only the 5-in-1 vaccine is included in the vaccination schedule (in 2008-2012, a 6-in-1 vaccine was also included). In Portugal, Finland, Norway, Montenegro and Bosnia and Herzegovina, only the Infanrix IPV+Hib vaccine is available. In Bosnia and Herzegovina, this vaccine has been available since 2012 in two of the three cantons.
In 15 countries, a hexavalent vaccine is available in the immunisation programmes. Infanrix hexa is available in Belgium, the Czech Republic, Latvia, Italy, Austria and the Netherlands. In Romania, the vaccinations are carried out using both 5- and 6-component vaccines. In Latvia, Pentaxim is available for children who receive a separate vaccine against hepatitis B; therefore, it is included in the immunisation schedule in addition to the 6-component vaccine Infanrix hexa. In Ireland, Slovakia, Switzerland and France, Infanrix hexa is in the vaccination programme along with Infanrix IPV+Hib. In Slovakia, Infanrix hexa is fully reimbursed when the child’s mother is HBsAg-positive7 . In Denmark, a 4-component vaccine produced by a domestic supplier is available [9].
. In Denmark, a 4-component vaccine produced by a domestic supplier is available [9].
The widest ranges of available vaccines against the analysed diseases are fully reimbursed in Greece, Spain and Germany. In Greece, there are both 5-component vaccines – Infanrix IPV+Hib and Pentaxim – and a 6-component vaccine Infanrix hexa. In Spain, the available vaccines include Infanrix IPV+Hib, Infanrix hexa and the pentavalent vaccine Pentavac (Pentaxim). In addition, there are plans to introduce the hexavalent vaccine Hexyon (Hexacima) in the Spanish market. In Germany, there are also three vaccines available, Infanrix hexa, Infanrix IPV+Hib and Hexacima (Hexyon). Hexacima is a new vaccine and the existing German system involves assessment of an innovative product after one year of market use, then a decision is made whether to continue funding the vaccine. This vaccine is available in Ireland, but it has not been introduced in the vaccination schedule yet, so it is currently not available free of charge, since Infanrix hexa will be reimbursed until 2016 as a result of a tender performed.
Figure 1. The availability of the analysed 5- and 6-valent vaccines in the national immunisation programmes across Europe
Source: own study; UMV, Universal Mass Vaccination
Funding and additional mechanisms to increase access to immunisation
In addition to providing the vaccine products in the public health care system, access to vaccines could be improved by the possibility of purchase on the private market, which means that the cost of purchasing the vaccine is fully covered by the parents/guardians of the child.
In countries such as Switzerland, Norway, Lithuania, Finland, Ireland, Belgium, Germany, Spain, there is one or more of the analysed vaccines available free of charge.
Another option is to provide access to a given vaccine or to more products as part of the national immunisation programme, and additionally allow the purchase of alternative products on the private market. An example is the Czech Republic where for several years the vaccination programme has been implemented using the 6-component vaccine Infanrix hexa, while the 5-component vaccine is available on the private market and the cost of its purchase is fully covered by the parents/guardians of the child as an alternative to Infanrix hexa. In Iceland, the vaccination programme includes Pentavac and it is the only vaccine available free of charge under the national immunisation programme, and from 2013, there is a possibility to purchase Infanrix IPV+Hib on the private market (in 2008–2012 it was available free of charge).
In Cyprus, the vaccination schedule includes a tetravalent vaccine Tetraxim (DTaP-IPV). These vaccines are available for the entire population visiting public hospitals (60% of the population of children); for the remaining 40% of the population of children, the 6-component vaccine can be purchased by the patient for the full payment during visits to private paediatric clinics. A similar situation is in Croatia, Slovenia and Slovakia, where in addition to free of charge vaccinations with 5- or 6-component products as part of the national programmes, the patient may choose to purchase a different vaccine for full payment.
If a product is not included in a universal vaccination schedule, the private market provides the only opportunity to purchase combination vaccines. This solution exists in the UK, Poland, Albania, Serbia, Malta, Cyprus and Macedonia. The planned start of Infanrix IPV+Hib reimbursement in Serbia is September 2013.
In Poland, combination vaccines are only available on the private market for full payment (multiple vaccines are not funded from the state budget); they are used by roughly 55% of parents8 . Also in Slovenia, Cyprus and Romania, Infanrix hexa can be purchased on the private market for full payment. The availability of new, innovative technologies depends, among other things, on the impact of the Health Technology Assessment agency on decision making regarding funding within the public system. The main factor influencing the rate of appearance of a product on the market is the role of health technology assessment reports in the health care system of a given country. For example, in Germany, the functioning mechanisms allow for public funding of medical technologies, in this case vaccines, from the time of their approval, and evaluation of the product is performed after a certain time. A different solution, which has been used, among others, in France and Sweden, is to conduct a health technology assessment before determining the price and the level of reimbursement. The longest average waiting time for product reimbursement was in Belgium and Portugal, and the shortest in Austria and Denmark, which directly affects the public availability of innovative medical technologies [10].
. Also in Slovenia, Cyprus and Romania, Infanrix hexa can be purchased on the private market for full payment. The availability of new, innovative technologies depends, among other things, on the impact of the Health Technology Assessment agency on decision making regarding funding within the public system. The main factor influencing the rate of appearance of a product on the market is the role of health technology assessment reports in the health care system of a given country. For example, in Germany, the functioning mechanisms allow for public funding of medical technologies, in this case vaccines, from the time of their approval, and evaluation of the product is performed after a certain time. A different solution, which has been used, among others, in France and Sweden, is to conduct a health technology assessment before determining the price and the level of reimbursement. The longest average waiting time for product reimbursement was in Belgium and Portugal, and the shortest in Austria and Denmark, which directly affects the public availability of innovative medical technologies [10].
In addition, another mechanism that allows the patient to choose the vaccine is to offer one product free of charge, and another product for partial payment; this is the case in Croatia, where Pentaxim is available free of charge, and Infanrix hexa can be purchased for a fixed price. In Belgium, co-financing is only available in exceptional cases, for older children which have not been vaccinated according to the recommendations. In this case, 25% of the vaccine price is covered by the patient.
Mechanisms of purchasing vaccines in the European health care systems
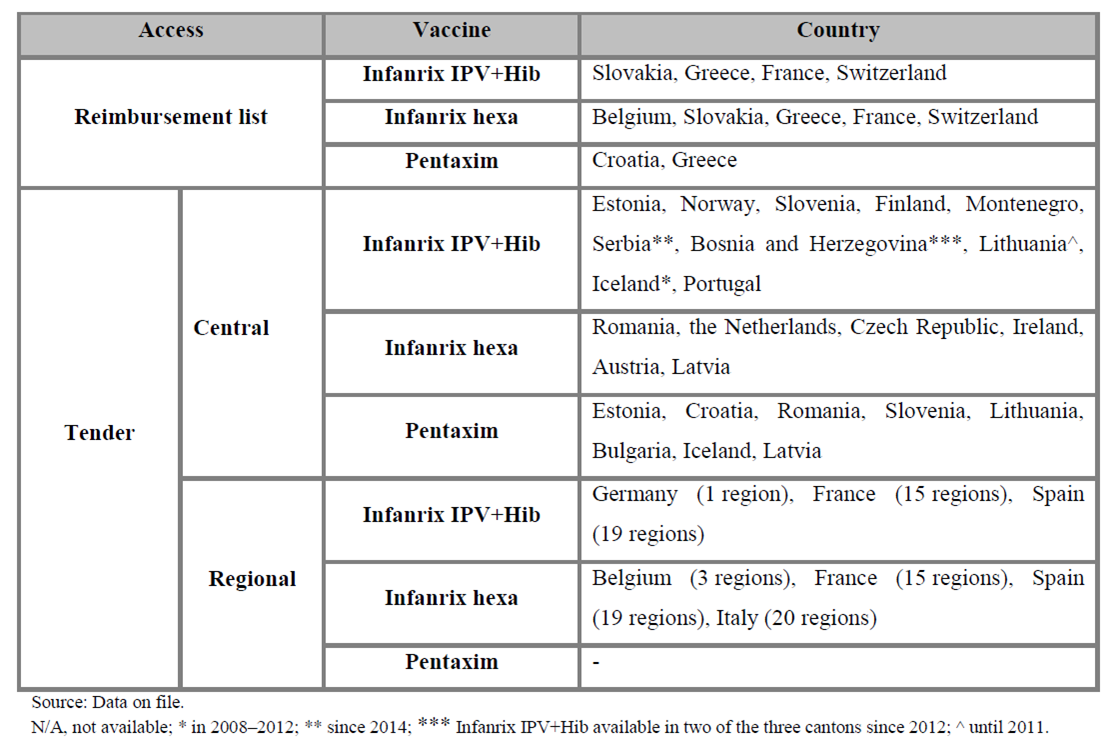
The mechanism of purchasing combination vaccines as part of the vaccination programmes is similar in the analysed countries. Purchase mainly occurs in the form of tenders, most commonly in a central tender. Only in five countries the purchase takes place in a regional tender – in Germany (1 region), Belgium (3 regions), France (15 regions), Spain (19 regions), and Italy (20 regions). In Germany, the situation is unusual, because only in one region the vaccine Infanrix IPV+Hib is available as a result of a won tender, while in the other regions the physician is free to choose from among the vaccines available on the German market.
Infanrix IPV+Hib is the most popular vaccine, which is the most widely available within the European public health care systems. In 2008 and 2009, as a result of a won tender, Infanrix IPV+Hib was available in 4 countries since 2008 or 2009 (the Netherlands, Norway, Finland, Montenegro). From 2012, this vaccine has also won a tender in Slovenia, and in Bosnia and Herzegovina. In Lithuania, in 2008–2010, both Infanrix IPV+Hib and Pentaxim were reimbursed; however, since 2011, the only vaccine selected by tender has been Pentaxim.
Infanrix hexa is also a universal vaccine, which has been available already for several years in the following countries: Italy, Ireland, Czech Republic, Austria, the Netherlands since 2011, and Romania since 2012. As part of a regional tender, both Infanrix IPV+Hib and Infanrix hexa have been available on the French market since 2008.
In the Netherlands, until August 2011, the vaccine chosen in a tender and available in the public health care system was Pediacel, and since 2011 it has been Infanrix hexa. Pediacel was also available in the years 2008–2010 in Croatia but since 2011 only Pentaxim has been available. Also in Bulgaria, Pentaxim was available free of charge in the vaccination schedule following a central tender in the years 2010 and 2012–2013. By 2012, only two or three kinds of vaccines were available in many countries as a result of a won tender. Currently Infanrix hexa and Pentaxim are publicly funded only in Romania, and both Infanrix IPV+Hib and Pentaxim are funded in Estonia and Slovenia. In Portugal, the vaccines are purchased by tender, and the government sets the price ceiling. In Cyprus, only the 4-in-1 vaccine has been available as a result of a central tender. However, in 6 countries the vaccines are included on the reimbursement lists, and therefore the target population has access to multi-combined vaccinations free of charge.
The mechanisms of purchasing the analysed vaccines based on the available data for the European countries are presented in the table below.
Places of patient access to vaccines
An important issue in the availability of vaccines is its place of purchase. The vaccines that are included in the immunisation programmes are delivered to the vaccination sites/medical practices; there are also no additional administrative costs associated with their administration.
In Belgium, Infanrix hexa may be available at pharmacies, but only in special cases not covered by the vaccination program. The vaccines can be purchased at the point of vaccination in Belgium, Poland and Italy. In Portugal, Finland, the Netherlands, Slovenia and Cyprus, children can receive a vaccine only at paediatric clinics (for healthy children), and in Lithuania, only in the primary health care institutions. In Romania, as part of the vaccination programme, combination vaccines are available in pharmacies and in the family doctors’ offices. In Poland, combination vaccines are available both at the points of vaccination and in pharmacies. In Germany, the patient receives a vaccine that has been included in the UMV directly from his/her doctor, and if a given product is not reimbursed by the health fund, then the patient can purchase the product for full payment in a pharmacy. In Switzerland, Infanrix hexa and Infanrix IPV+Hib, which are included in a UMV, are available for patients in paediatric practices; however, in a few regions, it is possible to purchase the vaccine in pharmacy with subsequent vaccination of the child by a paediatrician.
Paediatric vaccination schedules in Europe
The table below shows the regimen of administration of multiple vaccines in different countries. The analysis was performed for multi-component (5- or 6-component) vaccines, depending on the available data.
When analysing data from the schedules of vaccination with combination products, some differences can be seen in both dosing regimens and the time of administration. In the vaccination program in Austria and Serbia, the 6-component vaccine (DTaP-Hib-Hep-IPV) is given in a 2+1 vaccination schedule (at 3, 5 and 11–12 months of age). However, in Belgium it is administered in a 3+1 vaccination schedule (2, 3, 4 and 15 months of age). The 3+1 vaccination schedule has also been used in the Czech Republic, Germany and the Netherlands; the only difference is related to the booster dose administration time, namely in the Czech Republic, a booster dose is administered at 18 months of age, in Germany at 11–14 months of age, and in the Netherlands at 11 months of age (similarly to the 5-component vaccine available on the Dutch market for children born before 1 August 2011). The 3+1 vaccination schedule (2, 3, 4 months of age) is also approved for the 5-component vaccine (DTap-Hib-IPV) in Bulgaria and Hungary; the difference also relates to the last dose – in Bulgaria it is administered at 16 (not earlier than one year after administration of the 3rd dose), and in Hungary at 18 months of age. In Estonia and Slovenia, the 5-component vaccines are administered from the 3rd month of age, then in months 4 and 6, and the booster dose is given at 24 and 18 months of age, respectively (3+1 vaccination schedule). In Finland, Iceland, Norway and Croatia, the 5-component vaccine is administered in a 2+1 schedule at 3, 5 and 12 months of age, and in Portugal, Montenegro and the United Kingdom, at 2, 4 and 6 months of age. A similar dosing regimen is used in Ireland and Italy for the 6-component vaccine, which is administered at 2, 4 and 6 or at 3, 5–6 and 11–13 months of age, respectively. In Switzerland and Lithuania, immunisation with the 5-component vaccine is performed in a 3+1 vaccination schedule at 2, 4 and 6 months of age, and there is a difference in the booster dose which is given at 15–24 or 18 months of age, respectively. In Switzerland, the same scheme has been used for the 6-component vaccine. In the Netherlands and Spain, immunisation with both 5- and 6-components vaccines is given in a 3+1 vaccination schedule (at 2, 3, 4, 11 months of age and at 2, 4, 6 and 15–18 months of age, respectively), whereas in France it is administered according to a 2+1 vaccination schedule for both vaccines (2, 4, 16–18 months of age). In Romania, the 6-component vaccine is given at 2 and 6 months of age, and the 5-component vaccine is administered at 4 and 12 months of age.
Analysis of vaccination coverage
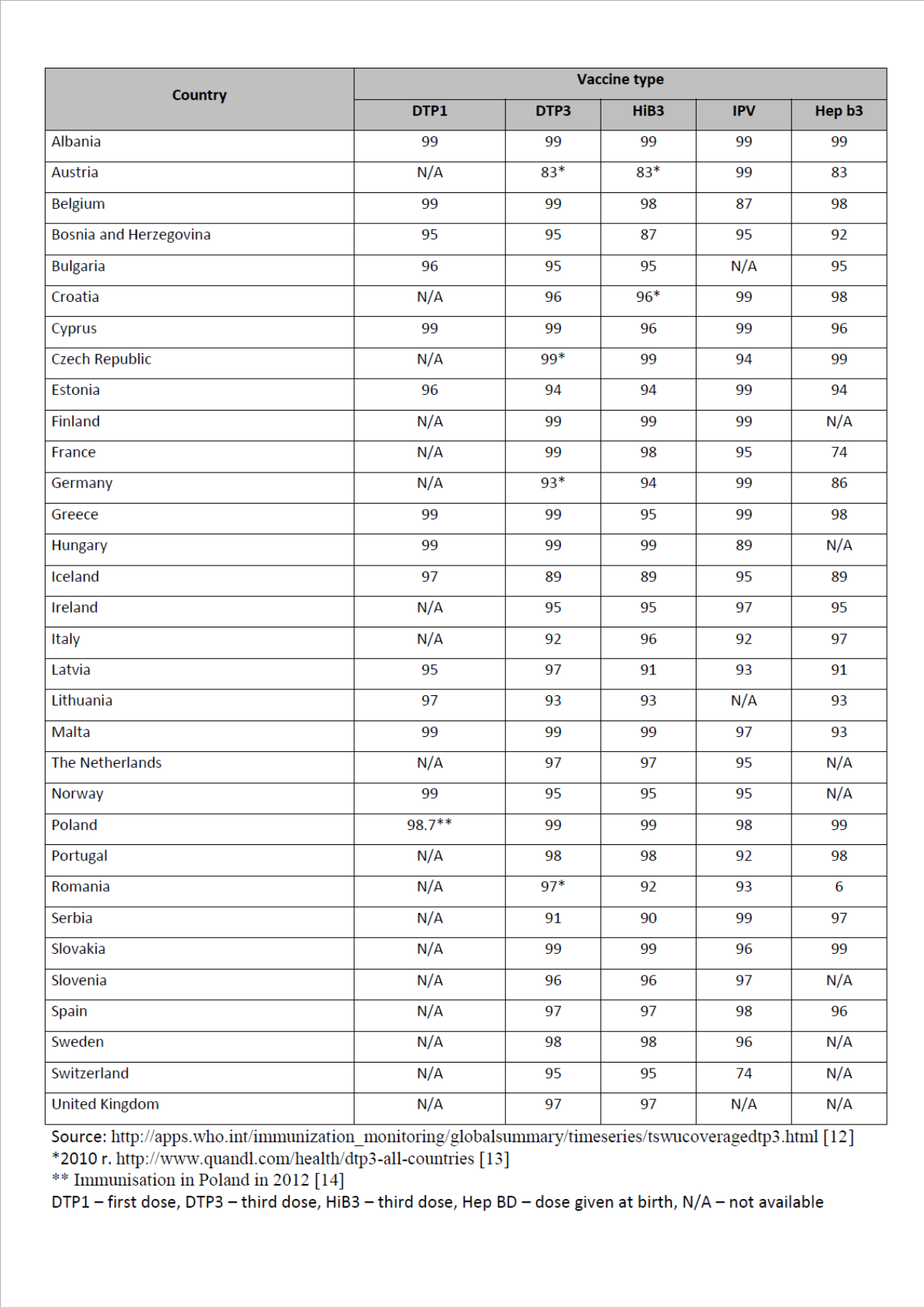
Based on the available European data for 2012 (last updated July 2013 [11]) it can be concluded that the level of vaccination rates against tetanus, diphtheria, pertussis, polio and Haemophilus influenzae type b invasive infections in most countries is at a high level, close to 95–99%. For hepatitis B vaccinations, data was available from 24 countries9 and the vaccination rates in most countries was high (96–99%). The lowest vaccination rate was reported for Austria (83%) and France (74%). A vaccination rate close to 100% (approximately 98–99%) against most diseases analysed in this study was seen in: Albania, Belgium, Cyprus, Hungary, Malta, Slovakia and Poland. The detailed data on the vaccination rate in each country in 2012 are presented in the table below.
and the vaccination rates in most countries was high (96–99%). The lowest vaccination rate was reported for Austria (83%) and France (74%). A vaccination rate close to 100% (approximately 98–99%) against most diseases analysed in this study was seen in: Albania, Belgium, Cyprus, Hungary, Malta, Slovakia and Poland. The detailed data on the vaccination rate in each country in 2012 are presented in the table below.
Table 4. Vaccination coverage rates against selected diseases in different European countries in 2012
Over the last 12 years (from 2000 to 2012), in the vast majority of countries there is a tendency to maintain high vaccination rate, or an increase in the level of vaccinated children. The data indicate that there has been no decrease in the childchood vaccination rate against the analysed diseases.
Discussion
Combination vaccines which represent pharmacological innovations have become increasingly commonly used over the years, and many countries have decided to reimburse them within the public health care system. The introduction in recent years of combination vaccines on the European market has provided a possibility of simultaneous immunisation against several diseases in a single injection.
Analysis of the data showed that the current immunisation programmes in Europe are not uniform. The differences mainly concern the time of administering the booster dose or the dosing regimen, which may result from different epidemiological conditions and vaccination funding opportunities in each country. Pentavalent vaccines are more common than hexavalent vaccines because in many countries the hepatitis B vaccine is still administered separately. In the vast majority of countries, there is a central system of purchasing vaccines as part of a central tender. This is the case of 28 of the analysed countries. Only in five countries (Germany, Italy, Belgium, France, Spain), the tenders are performed at the regional level. Based on these data it can be concluded that in 10 countries the tenders secured the supply of vaccines for several years (2–4 years). Data on the vaccination rate indicate that vaccines against diphtheria (D), tetanus (T), pertussis (Pa), hepatitis B (HBV), poliomyelitis (IPV) and Haemophilus influenzae type b (Hib) have been widely available and used. Analysis of the data showed that the percentage of vaccinated children is close to 100%, regardless of the product used and how it has been financed.
Conclusions
Combination vaccines are generally used for the prevention of infectious diseases. Their application, in addition reduces the number of injections required during the first two years of life children.
In the European national vaccine calendars the standard is recommending vaccination against: diphtheria (D), tetanus (T), pertussis (P), polio (IPV), Haemophilus influenzae type b (Hib), hepatitis B (HBV), so several years the vaccination rate remains very high. Along with the technological development, two-, three- or four-component vaccines are replaced by multi-combined vaccines (5-or 6-component), but the degree of their use in the national immunisation programmes and the level of their financing from public funds in different countries vary considerably.
In most countries, vaccination is recommended and reimbursed for the all population of children. The national immunization calendars for individual countries include age when child getting vaccine shot. If vaccinations are not available in national immunization programs, the only place where it is possible to obtain vaccines is the private market. In this case the cost of the vaccine is fully covered by the parents / guardians of the child.
It is reasonable conduct a further analysis of the European market in the private sector in terms of access is innovative vaccines.
Disclosures The study was sponsored by GlaxoSmithKline Pharmaceuticals SA
- Annual epidemiological report. Reporting on 2010 surveillance data and 2011 epidemic intelligence data 2012, ECDC
- Summary of Recommendations for Child/Teen Immunization (Age birth through 18 years). Available from: http://www.immunize.org/catg.d/p2010.pdf; [Accessed: 5.08.2013]
- Summary of WHO Position Papers – Recommended Routine Immunizations for Children. Available from: http://www.who.int/immunization/policy/Immunization_routine_table2.pdf; [Accessed: 5.08.2013]
- Summary of the European public assessment report (EPAR) for Infanrix Hexa. Available from: http://www.ema.europa.eu/docs/pl_PL/document_library/EPAR__Product_Information/human/000296/WC550003250.pdf; [Accessed: 5.08.3013]
- Infanrix penta 24 June 2010 EMA/378903/2013 Committee for Medicinal Products for Human Use (CHMP). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000295/WC500148294.pdf; [Accessed: 19.09.3013]
- Summary of the European public assessment report (EPAR) for Hexacima. Available from: http://www.ema.europa.eu/docs/pl_PL/document_library/EPAR__Summary_for_the_public/human/002702/WC500145811.pdf; [Accessed: 5.08.3013]
- http://www.urpl.gov.pl/system/drugs/dki/charakterystyka/2011-10-05_chpl_pentaxim_pkt_6_5_wersja_ostateczna.pdf; [Accessed: 19.09.3013]
- Available from: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con108611.pdf; [Accessed: 6.08.3013]
- Available from: http://www.ssi.dk/English/Vaccines/Bulk%20Vaccines%20and%20Toxoids.aspx; [Accessed: 6.08.3013]
- Wilsdon T., Serota A. A comparative analysis of the role and impact of Health Technology Assessment. CRA Charles River Associates Project No. D15891-00
- WHO Vaccine Preventable Diseases Monitoring System. Available from: http://apps.who.int/immunization_monitoring/globalsummary/; [Accessed: 6.08.3013]
- Available from: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragedtp3.html; [Accessed: 19.09.3013]
- Available from: http://www.quandl.com/health/dtp3-all-countries; [Accessed: 19.09.3013]
- Szczepienia ochronne w Polsce w 2012 roku. Narodowy Instytut Zdrowia Publicznego – Państwowy Zakład Higieny – Zakład Epidemiologii Główny Inspektorat Sanitarny – Departament Zapobiegania i Zwalczania Zakażeń i Chorób Zakaźnych u Ludzi. [Wstępne dane. 15.06.2013]