Mammography screening in the OECD and its impact on health and health system related indicators
-
Copyright
© 2014 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Sandra Wilde |
Hamburg University of Applied Sciences, Department Health Sciences |
|
Tanja Wirth |
Hamburg University of Applied Sciences, Department Health Sciences |
|
York Francis Zöllner |
Hamburg University of Applied Sciences, Department Health Sciences |
Background: Mammography screening, with its primary aim of breast cancer mortality reduction, is well implemented in most OECD member states. Overdiagnosis and overtreatment are often controversially discussed as potential consequences of screening. The objective of this study was to examine whether high mammography screening rates are associated with (a) higher incidence of and (b) lower mortality rates from breast cancer, and (c) higher inpatient mastectomy rates in OECD countries.
Methods: For this investigation, an ecological study design was chosen. Data of mammography screening rates, standardized incidence and mortality rates of breast cancer and inpatient mastectomy rates were derived from the database OECD.Stat Extracts for 2008 (or nearest year). Bar charts and scatter plots with associated R² were produced.
Results: Mammography screening rates showed a broad distribution among OECD states. Specific health indicators were, on average, less favorable in those countries where more women were screened. A high degree of variance explained by screening rates could be found for incidence rates of breast cancer and mastectomy rates (R²=0.522 and R²=0.258, respectively). For mortality rates, this was lower, but of medium size (R²=0.227).
Conclusion: Due to the ecological nature of the data, international variations in treatment guidelines and documentation of health indicators, the results must be interpreted with caution. However, the findings are in line with the contemporary literature. In the light of the observed correlation between mammography screening and less favorable health indicators, the role – whether explanatory or confounding – of potential overdiagnosis, overtreatment, and the time point of screening implementation remain controversial.
Introduction
Female breast cancer is the most prevalent neoplasm worldwide. In 2008, 5.2 million women were suffering from the disease [1], which is also the leading cause of death from cancer among the female population in Europe [2]. However, today it is believed that evidence-based screening tests, like mammography screening for breast cancer in women aged 50-69 years, followed by appropriate treatments, have the potential to prevent a large number of breast cancer deaths and, by that, reduce mortality rates of breast cancer [3]. Therefore, mammography screening programs, with the primary aim of breast cancer mortality reduction, have been implemented in most of the OECD countries during the last decade [4,5], in particular since the European Council recommended the implementation in December 2003 in all Member States [6]. After the adoption of screening, many studies have examined the effect of this diagnostic intervention on mortality rates and possible consequences, such as overdiagnosis and overtreatment, referring to the possibility that such breast cancers are diagnosed and treated which otherwise would have never posed a risk [4,7–10].
Nevertheless, to our best knowledge, the overall perspective of all OECD states concerning the association between mammography screening and health-related indicators has not been well studied in a comprehensive manner. Consequently, the aim of this investigation was to examine whether high mammography screening rates in females, aged 50-69 years, are associated with (a) higher incidence rates of breast cancer, (b) lower mortality rates from breast cancer, and (c) higher inpatient mastectomy rates, as a surrogate for subsequent health care activities, in OECD countries.
Materials and Methods
Data sources and definitions
For this investigation, an ecological study design was chosen, as aggregate data concerning mammography screening rates, incidence and mortality rates of breast cancer and mastectomy rates were available from the database OECD.Stat Extracts (http://stats.oecd.org/; February 21st, 2014).
Mammography screening rate was defined as the percentage of females aged 50-69 years being screened. For some OECD countries, this data was based on encounter data of a screening program, and for others, it was based on surveys [5]. Whenever information of both sources were available for a country, the encounter data was used, as it was assumed to be more accurate. Breast cancer was defined as malignant neoplasms of the female breast [ICD-10-CM code: C50). Age-standardized incidence rates (for the World Standard Population for 1960) [11] and age-standardized mortality rates (for the total OECD population for 2010) [12] of malignant neoplasms were included in the analysis per 100,000 females. Mastectomy rates were defined as inpatient mastectomy procedures per 100,000 females (ICD-9-CM code: 85.4) [13].
Data from the index year 2008 were included in the analyses. If no data for the index year were available, the method of last observation carried forward was applied (to a maximum of three years back, i.e. 2005).
In this study, all 34 OECD states were included initially. Poland, Spain and Sweden had to be eventually excluded from all analyses, as mammography screening rates – the main variable of interest in this study – were not available for these countries.
Statistical methods
Bar charts and medians with 25th and 75th percentiles as well as minimum and maximum percentages were presented to describe mammography screening rates among OECD states. Scatter plots with the corresponding R², as a measure of explained variance, were produced. This was used to estimate the bivariate correlation between mammography screening, which was determined as the independent variable, and various dependent variables (incidence rates, mortality rates and mastectomy rates; included separately). Exponential, linear and logarithmic regression models were tested and fitted, based on which of the three types showed the best explained variance of the two variables examined. For the interpretation of associations, a correlation coefficient, extracted root of R² in a bivariate analysis, between 0.1 - <0.3 was assumed to represent a small effect, between 0.3 – <0.5 a medium effect, and ≥ 0.5 a large effect [14]. Analyses were carried out using the Microsoft Excel 2007 spread sheet.
Results
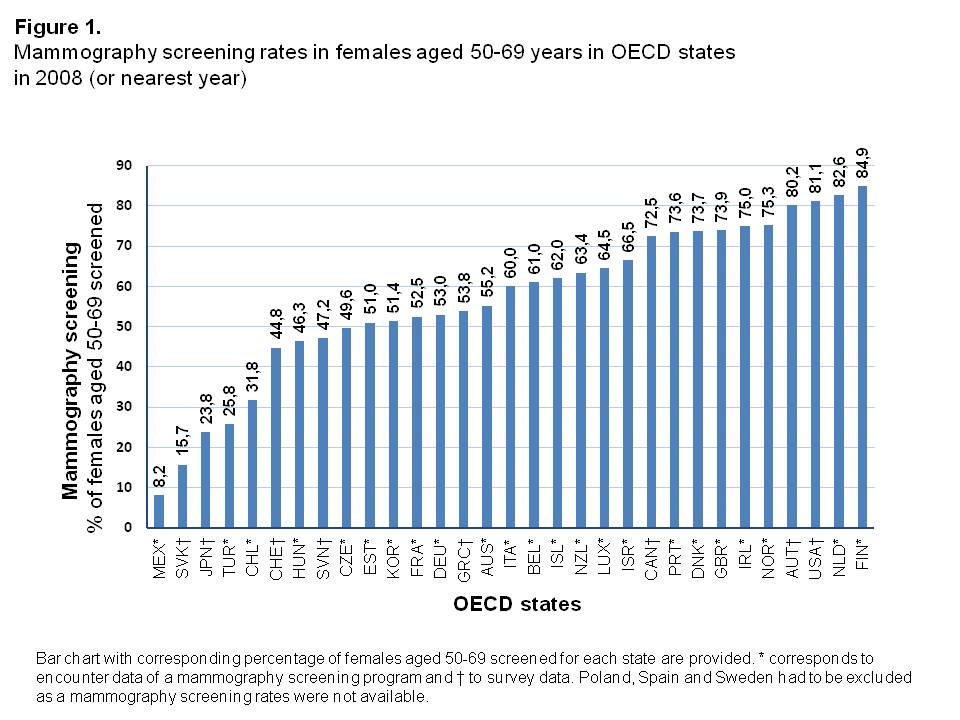
Mammography screening rates
Mammography screening rates in 2008 showed a considerably broad distribution among OECD states, from 8.2% in Mexico up to 84.9% in Finland (Figure 1, Supplementary Table 1). The median screening rate was 60.0% with a 25th Percentile of 48.4% and 75th Percentile of 73.7%.
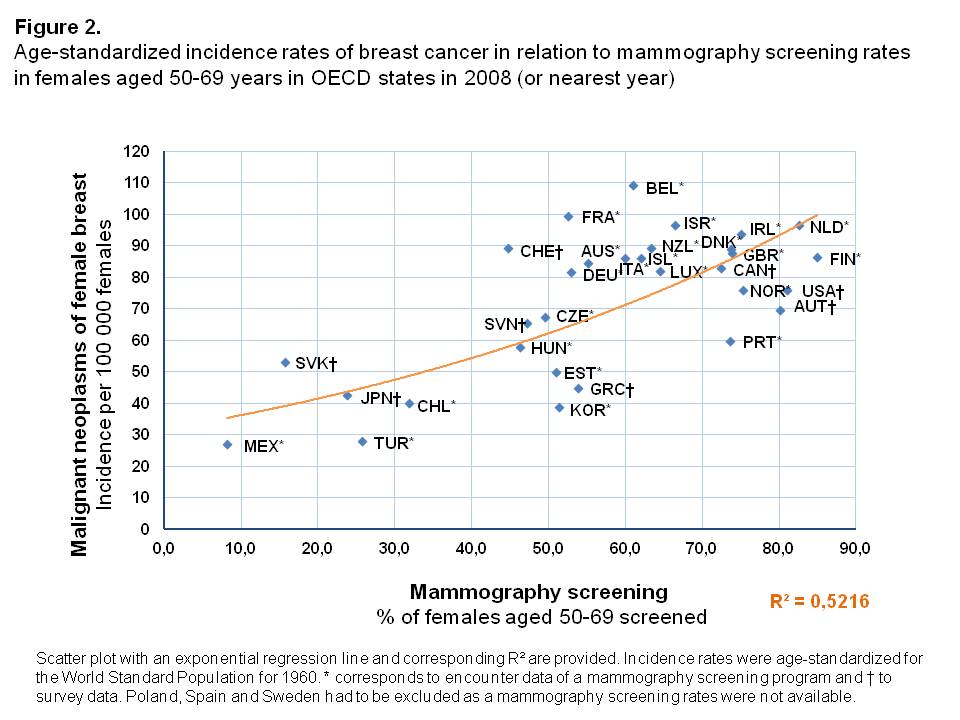
Incidence rates
In countries where a high proportion of the female population aged 50-69 years was screened, age-standardized incidence rates of breast cancer were higher than in OECD states where mammography screenings were less often performed (Figure 2). Overall, an exponential increase of incidence rates was found with a high degree of explained variance of R² = 0.522. Compared to countries with similar screening rates, the Slovak Republic was an outlier with a rather high age-standardized incidence rate (53.4 per 100,000 females), while only few women had undergone mammography screening in 2008 (15.7%).
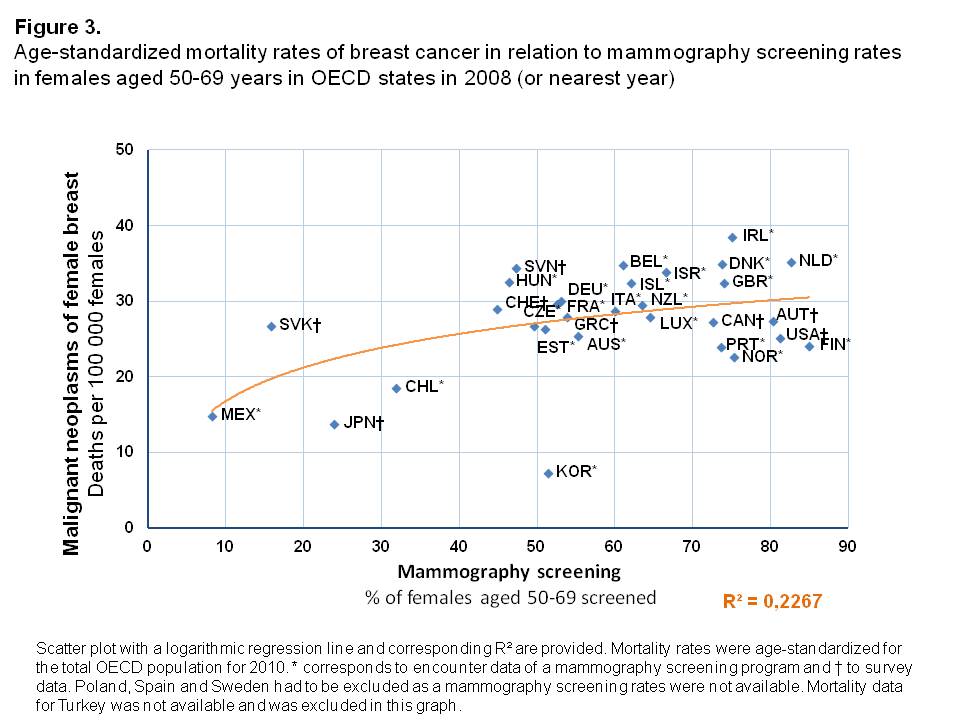
Mortality rates
The age-standardized mortality rates from malignant neoplasms of the female breast were higher in those OECD countries in which a larger share of the female population was being screened with mammography, shown by a logarithmic increase of mortality rates in relation to screening rates (Figure 3). The variance in the proportion of women screened could explain observed mortality rates from breast cancer to a medium degree (R²=0.227). Korea was an exception in this analysis. It was shown to have the lowest breast cancer mortality of all OECD countries (7.3 per 100,000 females), whereas a relatively high amount of women underwent mammography screening (Screening rate of 51.4%). Across all other countries with high mammography screening rates (>40%), there was only a small variation in mortality rates found. They were all in the range of 20-40 deaths per 100,000 females.
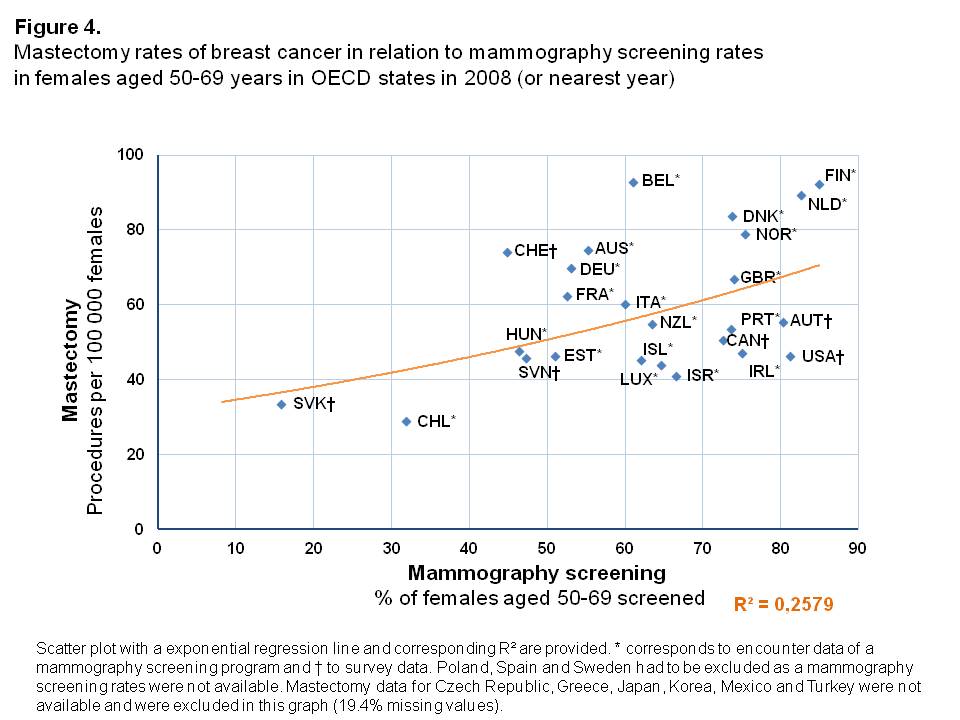
Mastectomy rates
Inpatient mastectomy rates were higher in OECD countries with higher percentages of women screened, with an exponential relationship between these two variables (Figure 4). The variance in mammography screening rates could explain, to a large degree, the observed mastectomy rates (R²=0.258). However, 6 of the 31 countries considered had to be excluded due to missing mastectomy rates in the period under study. Especially in the Netherlands and Finland, but also in Belgium, the mastectomy rates were high compared to OECD states with similar screening rates. Chile and Slovak Republic were outliers with rather low percentages of women screened and subsequently low inpatient mastectomy rates. Besides being outliers, both countries differ according to inpatient mastectomy rates and corresponding mammography screening rates. For Chile, the inpatient mastectomy rate is lower compared to the Slovak Republic (28.8 and 33.6 per 100,000 females, respectively), whereas the corresponding mammography screening rate is more than twice as high as in the Slovak Republic (31.8% and 15.7%, respectively).
Discussion
Discussion of methods
The international comparison of aggregate, macro-level data comes with methodological limitations which have to be kept in mind when interpreting the results.
The concept of ecological fallacy has to be mentioned in this context, as an ecological study design was used [15]. Results are based on aggregate data for 2008 (or nearest year); therefore, no assertions about causality or time trends regarding mammography screening and health or health system related indicators can be made, neither on national nor on individual level. Mammography screening rates were based on program or survey data, which are assumed to be more imprecise because of recall bias [5]. Hence, data acquisition is an additional bias. Furthermore, international variations in treatment guidelines and recommendations as well as definitions and documentation of malignant neoplasms influence incidence and mortality rates. Comparable ICD-10 and ICD-9 codes were used, which is an obvious advantage, but coding habits might vary across countries especially with regard to coding of the underlying causes of death [16]. Beyond that, different age-standardizations were used within the OECD data. Incidence rates were standardized for the World Standard Population of 1960, and mortality rates for the total OECD population of 2010, while inpatient mastectomy rates were not age-standardized at all. Due to these differences, as well as further diversities in medical culture, communication to patients, and selective access (e.g. via money or time prices borne by the patient) to screening tests across OECD countries, the conclusions to be drawn from the various measures of association between the rates studied and mammography screening are rather limited.
The strength of our analysis is the comprehensive comparison across OECD states. For most of the 34 OECD states, data were available, even if not all data for each indicator were available for the index year. The exercise was of exploratory nature, and the results found may be seen as a contribution to hypothesis-generation for bespoke study designs.
Discussion of results
Mammography screening rates vary across OECD states, which corresponds to former study results of variations across European countries [3]. The type of screening program, as in nationwide and/or additional opportunistic screening, or population-based vs. non-population-based, is strongly linked to variations in screening rates [3,17]. Apart from that, while the percentage of females screened is a key indicator of screening coverage (and hence access), the quality of administration and interpretation of mammography screening (screening interval, detection rates depending on technological sensitivity, specificity and specialization of staff) differs significantly between countries [3,5,7]. Mammography screening rates are strongly dependent on the phase of implementation of screening (pre-screening, introduction phase and fully-running program). Therefore, the comparison of screening programs as such across countries is challenging [17].
Overall, the type, quality and phase of implementation have important implications on health and health system related indicators.
Concerning breast cancer incidence, an increase of this rate is often associated with the introduction phase of a novel mammography screening program [4,17]. During the course of a screening program, detection rates are expected to decrease again after a certain run-in phase [17]. Therefore, different phases of implementation in the OECD countries could explain the broad distribution of breast cancer incidence rates. Furthermore, the incidence is also strongly dependent on risk factors of the disease, such as genetic predisposition, increased exposure to hormones, overweight and alcohol consumption, which vary widely across the countries under study [5]. The exponential increase of incidence rates in relation to mammography fits the popular notion of “when more is searched, more is found” or, on a scientific level, the controversially discussed allegation of a potential overdiagnosis of breast cancer as a result of wide screening [4,7,18–20]. It is argued that through screening, many slow-growing tumors with a long non-symptomatic phase are detected which would otherwise not have been found in the remaining life-span of the individuals concerned, and would never have been fatal [9]. A systematic review of randomized controlled trials assumed a rate of overdiagnosis of about 30% [7]. Recently, in a randomized screening trial in Canada, it was found out that 22% of screen-detected invasive breast cancers were over-diagnosed, corresponding to one over-diagnosed case in 424 women screened. However, data collection started in the 1980s, and mammography screening at that time might not be comparable to modern standards [8]. An Independent UK Panel on Breast Cancer Screening also warned that estimates of overdiagnosis are subject to several uncertainties, and only rely on small amounts of data. Although the Panel conceded that overdiagnosis may occur, they found the most reliable estimates of overdiagnosis in women invited to screening to be 11% of cancers diagnosed during lifetime, and 19% of cancers diagnosed during screening programs [19]. In addition, as the severity of tumors was not assessed in this study, it can only be suspected that overdiagnosis of small invasive breast cancers and in situ lesions contribute to higher incidence rates in OECD states, where more women are screened.
In our study, it was found that mortality rates are logarithmically increasing with respect to screening rates, which is in contrast to the hypothesis that, in OECD states with higher screening rates, mortality from breast cancer is lower. This could be explained by the “sticky diagnosis bias”: Due to mammography, the number of women diagnosed with breast cancer increases; subsequently, mortality rates inflate, because the diagnosis might “follow” the individual and influence decisions regarding coding of the underlying cause of death [4]. Additionally, screening might also increase mortality via more performed radiotherapies, which are said to be harmful for women with a low risk of local recurrence, which often applies to tumors found by mammography [7]. Nevertheless, current research suggests a decrease of mortality rates by screening of 15% [7], 26% after 6-11 years of follow-up [10], or even up to 48% [21]. An explanation for these varying results might be, apart from methodological issues, that studies usually compared mortality rates before and after the implementation of a screening program. With aggregated data used in this study, it was only possible to compare the rates in countries during one specific year, which might explain the contrasting findings. Moreover, mortality reduction at population level is expected to occur, at the earliest, several years after implementation of mammography programs, and is also depending on the implementation phase [17]. This might be also the reason for a particularly low mortality rate in Korea. An increase of the mortality rate is expected as a result of an increasing incidence rate in Korea within the last years, maybe due to the adaption of western lifestyles. In turn, an increase of the mortality rate at population level will be seen several years afterwards [22]. Contrasting to these findings, it was concluded in the current literature, based on results of a recent randomized screening trial, that mammography did not achieve to reduce mortality from breast cancer for women aged 40-59, and by that, authors recommended to reassess the rationale for mammography screening [8].
Mastectomy rates seem to be exponentially higher in OECD countries with higher mammography screening rates, which corresponds to the hypothesis that activity will follow diagnosis, although data was incomplete for mastectomy rates of the respective countries. Very much like incidence rates, mastectomy rates depend on the stage of screening implementation; whereby mastectomy rates are expected to be higher during implementation and are likely to decline during fully-running screening programs [17]. This might have influenced the differences in mastectomy rates. Besides, variation in mastectomy rates can be a result of diverse national interventional policies (e.g. treatment guidelines and recommendations of therapy) independently from screening policies [17,23]. Related to this, changes in such guidelines, e.g. from mastectomy as the standard treatment towards breast-conserving therapy, are important to take note of (and correct for), as they may differ across OECD states [17,24]. For example, a lower decrease of mastectomy rates during the mammography screening implementation in Germany might be due to a higher proportion of breast-conserving surgeries as a new health policy [17]. In analogy to overdiagnosis, overtreatment – which means ‘aggressive’ therapy of tumors which would have never posed a risk [4] – is a further possible reason for higher mastectomy rates in states with higher screening rates. A Cochrane review of randomized trials pointed out that mastectomy rates increased by 20% in women who underwent mammography screening, compared to those who were not screened [7]. For Denmark, which also featured high mastectomy rates in our analysis, 33% overdiagnosis and overtreatment was reported, which is still lower than previously expected [9].
However, in the case of Germany, reliable data on the effectiveness of mammography screening with regard to decrease of breast cancer mortality are not yet available, and are expected to be published in five to seven years [25]. For the UK, the Independent Panel weighs in significant benefits such as an estimated 20% reduction in overall mortality in women invited to a 20-year screening program, against possible harms of diagnosis and treatment of cancer that would never have caused problems, concluding that the screening program should continue, with the proviso that the pros and cons need to be clearly communicated to women [19].
Conclusions
Due to ecological design used and international variations in definitions, documentation, and guidelines to name but a few, the interpretation of the findings, i.e. the associations shown, needs to be handled with extreme caution. However, our results are in line with much of the current body of the literature. As potential reasons for less favorable levels of specific health indicators in relation to mammography screening, the roles of overdiagnosis, overtreatment as well as the phase of screening implementation can be discussed controversially. Regarding mastectomy rates, one must conclude that variation among OECD states might be partly be independent of screening coverage and due to national health policies, which are themselves prone to differ across OECD states, and even within the same states over time. Contrary to an intuitive hypothesis, mortality rates seem to be higher in OECD states with higher screening coverage. This could be biased or confounded by several factors, one of which is that mortality is expected to decrease in the general population only several years after implementation of a screening program. Therefore, ongoing research is necessary to assess the harm-benefit balance based on data from modern and nationwide mammography screening programs.
Supplementary Table 1.
Mammography screening rates in females and corresponding health and health system-related indicators in OECD states in 2008 (or nearest year)
|
OECD states |
Mammography screening rate |
Incidence rate |
Mortality rate |
Mastectomy rate |
|
% of females aged 50-69 screened |
Malignant neoplasms |
Malignant neoplasms |
In-patient procedures |
|
|
Australia* |
55.2 |
84.8 |
25.5 |
74.6 |
|
Austria† |
80.22 |
69.9 |
27.4 |
55.5 |
|
Belgium* |
61.02 |
109.4 |
34.8 |
92.9 |
|
Canada† |
72.5 |
83.2 |
27.3 |
50.5 |
|
Chile* |
31.81 |
40.1 |
18.6 |
28.8 |
|
Czech Republic* |
49.6 |
67.7 |
26.8 |
--- |
|
Denmark* |
73.7 |
89.1 |
34.9 |
83.6 |
|
Estonia* |
51.0 |
50.2 |
26.3 |
46.2 |
|
Finland* |
84.9 |
86.6 |
24.1 |
92.2 |
|
France* |
52.5 |
99.7 |
29.7 |
62.4 |
|
Germany* |
53.0 |
81.8 |
30.1 |
69.8 |
|
Greece† |
53.8 |
44.9 |
27.9 |
--- |
|
Hungary* |
46.3 |
57.9 |
32.6 |
47.6 |
|
Iceland* |
62.0 |
86.2 |
32.5 |
45.2 |
|
Ireland* |
75.0 |
93.9 |
38.6 |
47.2 |
|
Israel* |
66.5 |
96.8 |
33.9 |
40.9 |
|
Italy* |
60.0 |
86.3 |
28.8 |
60.3 |
|
Japan† |
23.81 |
42.7 |
13.8 |
--- |
|
Korea* |
51.4 |
38.9 |
7.3 |
--- |
|
Luxembourg* |
64.5 |
82.3 |
28.0 |
44.0 |
|
Mexico* |
8.2 |
27.2 |
14.9 |
--- |
|
Netherlands* |
82.6 |
96.8 |
35.2 |
89.4 |
|
New Zealand* |
63.4 |
89.4 |
29.5 |
54.9 |
|
Norway* |
75.3 |
76.2 |
22.6 |
78.8 |
|
Portugal† |
73.63 |
60.0 |
24.0 |
53.4 |
|
Slovak Republic* |
15.7 |
53.4 |
26.8 |
33.6 |
|
Slovenia† |
47.21 |
65.5 |
34.5 |
45.8 |
|
Switzerland† |
44.81 |
89.4 |
29.0 |
74.2 |
|
Turkey* |
25.8 |
28.3 |
|
--- |
|
United Kingdom* |
73.9 |
87.9 |
32.5 |
66.9 |
|
United States† |
81.1 |
76.0 |
25.2 |
46.3 |
Data were available for 2008 or nearest year (1 2007; 2 2006 and 3 2005). * corresponds to program data and † to survey data. Poland, Spain and Sweden had to be excluded as a mammography screening rates were not available.
- 1. Bray F, Ren J-S, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–45
- 2. Ferlay J, Parkin D, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. Elsevier Ltd; 2010;46(4):765–81
- 3. Karsa L von, Anttila A, Ronco G, Ponti A, Malila N, Arbyn M, et al. Cancer screening in the European Union. Report on the implementation of the Council Recommendation on cancer screening. Luxembourg: European Communities; 2008
- 4. Jørgensen K. Mammography screening. Benefits, harms, and informed choice. Dan Med J. 2013;60(4):1–26
- 5. OECD. Health at a Glance 2011: OECD Indicators. OECD Publishing; 2011
- 6. Puliti D, Zappa M. Breast cancer screening: are we seeing the benefit? BMC Med. 2012;10:106
- 7. Gøtzsche P, Nielsen M. Screening for breast cancer with mammography (Review). Cochrane Libr. 2009;(4):1–70
- 8. Miller A, Wall C, Baines C, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348(g366):1–10
- 9. Jørgensen KJ, Zahl P-H, Gøtzsche PC. Overdiagnosis in organised mammography screening in Denmark. A comparative study. BMC Womens Health. 2009;9:36
- 10. Njor S, Nyström L, Moss S, Paci E, Broeders M, Segnan N, et al. Breast cancer mortality in mammographic screening in Europe: a review of incidence-based mortality studies. J Med Screen. 2012;19 Suppl 1:33–41
- 11. OECD. OECD Health Data 2012 Definitions , Sources and Methods Cancer [Internet]. 2012 [cited 2014 Apr 8]. Available from: http://stats.oecd.org/OECDStat_Metadata/ShowMetadata.ashx?Dataset=HEALTH_STAT&Coords=%5bVAR%5d.%5bCANCBREC%5d&ShowOnWeb=true&Lang=en
- 12. OECD. OECD Health Data 2012 Definitions , Sources and Methods - Causes of mortality [Internet]. 2012 [cited 2014 Apr 8]. Available from: http://stats.oecd.org/OECDStat_Metadata/ShowMetadata.ashx?Dataset=HEALTH_STAT&Coords=%5bVAR%5d.%5bCICDMNBR%5d&ShowOnWeb=true&Lang=en
- 13. OECD. OECD Health Data 2012 Definitions , Sources and Methods - Surgical procedures by ICD-9-CM [Internet]. 2012 [cited 2014 Apr 8]. Available from: http://stats.oecd.org/OECDStat_Metadata/ShowMetadata.ashx?Dataset=HEALTH_PROC&Coords=%5bVAR%5d.%5bVARPMAST%5d&ShowOnWeb=true&Lang=en
- 14. Field A. Correlation. In: Field A, editor. Discovering Statistics Using SPSS. 4th Editio. London: Sage Publications; 2013. p. 262–90.
- 15. Gordis L. Epidemiology. Clemens S, Schäfer T, Groos J, editors. Marburg: KILIAN Verlag; 2001
- 16. Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83(3):171–7
- 17. Stang A, Kääb-Sanyal V, Hense H-W, Becker N, Kuss O. Effect of mammography screening on surgical treatment for breast cancer: a nationwide analysis of hospitalization rates in Germany 2005-2009. Eur J Epidemiol. 2013;28(8):689-696
- 18. Biller-Andorno N, Jüni P. Abolishing mammography screening programs? A view from the Swiss Medical Board. N Engl J Med. 2014;370(21):1965–7
- 19. Marmot M. The benefits and harms of breast cancer screening: an independent review. Lancet. Elsevier Ltd; 2012;380(9855):1778–86
- 20. Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311(13):1327–35
- 21. Broeders M, Moss S, Nyström L, Njor S, Jonsson H, Paap E, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19 Suppl 1:14–25
- 22. Son BH, Kwak BS, Kim JK, Kim HJ, Hong SJ, Lee JS, et al. Changing patterns in the clinical characteristics of Korean patients with breast cancer during the last 15 years. Arch Surg. 2006;141(2):155–60
- 23. Allemani C, Storm H, Voogd AC, Holli K, Izarzugaza I, Torrella-Ramos A, et al. Variation in “standard care” for breast cancer across Europe: a EUROCARE-3 high resolution study. Eur J Cancer. 2010;46(9):1528–36
- 24. Suhrke P, Mæhlen J, Schlichting E. Effect of mammography screening on surgical treatment for breast cancer in Norway : comparative. BMJ. 2011;343:1–8
- 25. Deutsches Ärzteblatt. “Die Sensitivität des Mammopgraphie-Screeningprogramms ist gut” [Internet]. 2014 [cited 2014 Jul 25]. Available from: http://www.aerzteblatt.de/nachrichten/59496/Die-Sensitivitaet-des-Mammographie-Screeningprogramms-ist-gut