Risk of severe hypoglycaemia for various treatment regimens – a systematic review and meta-analysis of observational studies
-
Copyright
© 2014 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Michał Jakubczyk |
Institute of Econometrics, Warsaw School of Economics, Poland |
|
Justyna Pawęska |
HealthQuest spółka z ograniczoną odpowiedzialnością Sp. K, Warsaw, Poland |
|
Maciej Niewada |
Department of Experimental and Clinical Pharmacology, Medical University of Warsaw, Poland |
|
Elżbieta Rdzanek |
HealthQuest spółka z ograniczoną odpowiedzialnością Sp. K, Warsaw, Poland |
|
Marcin Czech |
Novo Nordisk Pharma sp. z o.o. |
Background: Previous publications show that diabetes mellitus (DM) is a grave medical and economic problem, largely due to complications. The objective is to evaluate real-life risk of severe hypoglycaemic events (SHEs) among diabetic patients (type 1 and 2, T1&2) for various therapies.
Methods: We conducted a systematic review of observational studies in MEDLINE, Embase, and The Cochrane Library databases. Observational, retrospective or prospective, studies (with at least 100 participants) in children and adults were included, with focus on: time horizon, number of patients, number of SHEs, and number of patients experiencing SHEs.
In T1 DM we distinguished basal-bolus/pre-mix insulin and insulin pump, and in T2 DM we singled out basal-bolus/pre-mix insulin, basal supported oral therapy with insulin as the basal component, sulfonylurea, and other antidiabetic medications.
We used a Poisson model implemented in Bayesian framework in WinBugs to estimate the SHE.
Results: We identified 55 relevant studies encompassing 245,028 patients (103,741.81 patient-years). Annual SHE rates varied in T1DM from 0.18 (95%CI: 0.13–0.25) for insulin pump up to 1.1 (0.57–2.71) for basal-bolus with human basal insulin, and in T2DM from 0.006 (0.001–0.008) for oral antidiabetic drugs (excl. SU) up to 0.56 (0.16–9.65) for basal-bolus with human insulin as the basal component.
Conclusions: Our results confirm that available treatment regimens differ in SHEs risk in real-life setting. Still SHEs are also driven by other factors, e.g. lifestyle, which may impact treatment selection.
Background
Not only is diabetes mellitus (DM) an expensive medical condition, but it is also a multidimensional one, leading to wide range of complications that themselves may be clinically important or associated with high resource consumption. One of these is hypoglycaemia, that is often related to antidiabetic drugs and might affect patients compliance, quality of life and treatment outcomes. Most of hypoglycaemic events are not documented, however severe hypoglycaemic events (SHEs) require assistance of another person, and can be even fatal, although rarely. Antidiabetic drugs are associated with various rates of hypoglycaemia, and the burden of hypoglycaemia is determined mainly by drug use patterns and patients’ adherence, but also diet and exercise. A review of the importance of hypoglycaemia from the perspective of the clinical process (clinical inertia, patient’s adherence) and the list of possible causes and risk factors can be found e.g. in Ahrén [1].
Hypoglycaemia is now being frequently used in cost-effectiveness modelling in DM [e.g. 2,3] and often constitutes an important part either strongly influencing the resulting incremental cost-effectiveness ratios [e.g. 4,5] or being an outcome measure [e.g. 6]. Hypoglycaemia has also been subject to cost-of-illness studies, e.g. Jönsson et al. [7] for T2 DM in Sweden. The body of evidence in such studies is limited as–to the best of our knowledge–no systematic review and meta-analysis of severe hypoglycaemia risk has been performed. E.g. in their study Jönsson et al. assumed the rates of SHE based on five studies only [8-12]. The above observations motivate our research to try to estimate real-life risk of SHE based on best available evidence. The aim of the present study is to collect real-life data on absolute number of hypoglycaemic events in order to evaluate risk of SHE among patients with DM using various treatment regimens. These estimates can then be used e.g. in cost studies or to populate economic models on DM and its complications.
In order to make the estimates as close to real-life settings as possible, we decided to use observational studies only and not randomized controlled trials (RCTs). Importantly our goal was to assess the absolute risk of SHE in an observational, rather than interventional, context, i.e. we want to assess what the risk of hypoglycaemia is when we observe a patient to use a given therapy, and not when we prescribe a given therapy to patient. In real-life clinical practice many factors influence the treatment selection in DM, baseline risk of SHE being probably one of them. That is why a problem of confounding would appear when trying to interpret our results (obtained from observational studies) in interventional manner. Thus, for our purpose observational studies are more relevant than RCTs. It is also important to stress that our results ought not to be used to compare treatments between each other to see what the results of replacing one treatment by another would be. Therefore we did not present relative rates.
As there are numerous drugs that can be used in DM, some grouping is necessary, as otherwise the body of evidence for each individual treatment would be too small to make credible inferences, and random errors would drive the results. That is why we decided to group all possible treatment regimens in a dozen of categories (4 in T1, 8 in T2) based on clinical guidelines and consultation with clinical experts.
The paper is structured as follows. In the next section we present the methodology of our systematic review. We present the search strategy and criteria used, as well as assumptions made in meta-analysis of the data. We then present results in section 3. These encompass the results of our systematic review of observational studies and of a review of secondary studies that was used to fill in the gap when primary studies were unavailable for some regimens. We also present the resulting estimates of SHE rates for analysed regimens. We discuss the findings and limitations in section 4 and briefly conclude in the last section.
Methods
We analysed SHEs in type 1 and type 2 (T1&T2) DM patients. We used SHE definition proposed by Jönsson et al. [7] i.e. an event of low plasma glucose level when a patient requires help from another person to manage, as this definition directly relates to resource usage.
Based on the anticipated different drug related SHEs risk we defined the following treatment groups. In T1 DM: insulin pumps, basal-bolus insulin therapy with long-acting insulin analogue as the basal component (BBA), basal-bolus insulin therapy with human insulin as the basal component (BBH), biphasic insulin analogue, biphasic human insulin. In T2 DM: sulfonylurea (SU) with or without other oral drugs but excluding insulin, other antidiabetic medications especially oral antidiabetic medications different than SU (OADs excl. SU), basal long-acting insulin analogue (BOTA), basal human insulin (BOTH), basal-bolus with long-acting insulin analogue as the basal component (BBA), basal-bolus with human insulin as the basal component (BBH), biphasic insulin analogue, biphasic human insulin (all insulin regimens could be in combination with OADs). We defined basal bolus insulin therapy as long acting insulin analogue once or twice daily and short/ultrashort insulin at mealtime (BBA).
Although SR did not have a registered protocol, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. As we wanted to assess SHEs rates in real-life rather than in experimental settings we looked for observational studies in: MEDLINE, Embase and The Cochrane Library databases (search strategies are given in Online Resource ESM_1). To account for changes in clinical practice in recent years and possible impact on treatment related risks, only recent studies were included (newer than 10 years).
We limited our search strategies to insulins or SU (i.e. we used no specific keywords for other-than-SU oral antidiabetic medications). We took this approach as NICE, IDF, ADA and EASD guidelines [7,14-17] firmly indicate that among oral antidiabetic medications used for treatment of T2 diabetes sulfonylureas are associated with an increased risk of hypoglycaemia as compared to other drug groups. The risk of hypoglycaemia associated with GLP-1 agonists and DPP-4 inhibitors is similar and very low [18,19]. Hence we treated GLP-1 agonists and OADs other than SU as one group, associated with a similar and most likely negligible SHEs risk. We assumed that the estimate of the risk of hypoglycaemia will use the best data found for one of these drugs. We decided to narrow the primary search then and to asses SHE rate in this group by applying a relative rate found in the literature as compared to SU, as described in more details below.
Precisely, specific inclusion criteria for observational studies encompassed: i) population of children and adults with T1 or T2 diabetes; ii) study design, i.e. observational, retrospective or prospective; iii) at least 100 participants (in total in a study, possibly split into smaller subgroups); iv) assessment of SHEs defined as an episode when the patient required an assistance from another person; v) publication date from 1st January 2002 until the search date, i.e. 1st October 2012.
Two authors independently conducted the selection process of relevant trials. Protocol assumed that in case of discrepancies between the authors discussion would be held until consensus was reached.
To estimate SHEs rates various types of data had to be extracted: time horizon in which hypoglycaemia was assessed, number of patients in a study group, number of hypoglycaemic episodes (absolute or mean per patient in a specified period of time, if available), number of patients experiencing at least one SHE (if available). If one study was described in many manuscripts, then the ones with the most appropriate and complete results were selected for extraction (e.g. data for a total study cohort instead of subpopulation, results presented separately for patients with T1 and T2 diabetes or results split by insulin regimens of interest). Data from included studies were extracted by one of the reviewer and checked by the other one.
As mentioned above, we planned to assess the risk related to other antidiabetic medications – GLP-1 or OADs (excluding SU) for T2 DM, calculating the relative rates as compared to SU based on secondary studies and then imposing them on the background SU-related SHE rate. We looked for the relative rates in secondary studies (SRs, meta-analyses) searched in a systematic way (see Online Resource ESM_1 for a search strategy) in MEDLINE, Embase, The Cochrane Library and Centre for Reviews and Dissemination (CRD). Inclusion criteria for this additional search encompassed: i) search performed at least in two databases (including at least one of the above databases), ii) at least two authors, iii) description of search strategy, iv) inclusion of randomized controlled trials (RCTs) conducted on T2 DM with at least one of the following: dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 agonist, other oral antidiabetic drugs i.e. metformin, TZD, v) with hypoglycaemia defined as an episode when a patient required help form another person. We decided to use RCTs as they are more common to provide data on relative rates (than observational studies).
Our systematic review of primary studies yielded no studies in T1 DM patients treated with biphasic insulins. We thus had to update our methods and we conducted a supplementary literature search for secondary studies. We applied a similar methodology as with OADs, i.e. we looked for systematic reviews of RCTs in T1 DM patients treated with premixed insulins. We then applied relative rates to assess absolute rates.
We wanted eventually to asses annual SHEs rates per one person, i.e. average number of SHEs per one patient-year of staying on therapy. We assumed a random effects model, i.e. assumed that mean rates per treatment regimen in individual studies are drawn from some distribution, whose average we aim to estimate. We assumed that number of SHEs in individual patient follows a Poisson distribution, which allowed to use the information on both the average number of SHEs in a study and the fraction of patients with at least one SHE in a given horizon. Our model was expressed in Bayesian framework and implemented in WinBugs (see Online Resource ESM_2). Random effects model and non-informative priors were used. Median from a posterior distribution was used as a point estimator, and 2.5% and 97.5% percentile defined a 95% Bayesian confidence interval.
Risks related with other ADs were assessed in a two-step procedure. First a relative rate between other ADs and SU was assessed based on RCTs using fixed effect model in WinBugs. It was then applied to the baseline rate estimated for SU from observational studies.
We assessed the quality of included studies using the Newcastle-Ottawa Scale [20] – for case-control and cohort studies. According to systematic review by Deeks et al. [21], this scale is one of the two best identified for evaluating non-randomised interventional studies and is suitable for use in a systematic review (either as a scale or a checklist). Moreover, this tool is mentioned in the Cochrane Handbook as a tool for assessing methodological quality or risk of bias in non-randomized studies [22]. Non-interventional studies of other types were assessed by focusing in methods of patients selection, methods of outcome recording, study size and study representativeness.
Results
Systematic review of observational studies
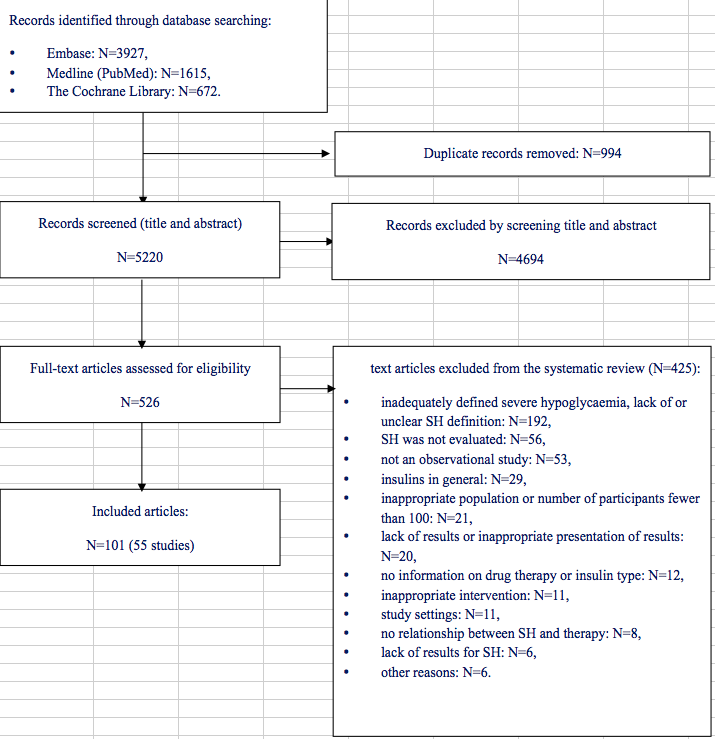
Literature search (for primary studies) yielded 6214 records, from which 994 duplicates were removed. The remaining 5220 articles were screened by title and abstract, and then 526 full texts were reviewed. Finally, 101 manuscripts [23-123] describing 55 individual trials were assessed as eligible for the analysis. Fig. 1. shows the studies selection process. Characteristics of included studies and references to the excluded studies with justifications are given in Online Resources ESM_3 and ESM_4, respectively.
For T2 DM 76 articles describing 35 studies were included: 11 (11 278.88 patient-years in total) provided data on BOT with insulin analogue; 7 (2142.13 patient-years in total) – BOT with basal human insulin; 6 (3022.27 patient-years in total) – BB with basal insulin analogue; 3 (227.46 patient-years in total) – BB with basal human insulin; 12 (63 776.85 patient-years in total) – pre-mixed insulin analogues; 6 (2265.87 patient-years in total) – pre-mixed human insulin ; 6 (1776.00 patient-years in total) – sulfonylureas.For T1 DM 33 articles describing 21 studies were included: 14 (6714.61 patient-years in total) provided data on SHEs in patients on insulin pumps, 7 (9656.18 patient-years in total) – BB with insulin analogue as the basal component, and 6 (2881.57 patient-years in total) – BB with human insulin as the basal component. As mentioned above, no studies on the treatment with biphasic insulins in T1 diabetes were found. A supplementary search for studies on pre-mixed insulins in T1 was carried out using the following key words: “biphasic”, “pre-mix”, “insulin”, “type 1” and “diabetes” and resulted in six systematic reviews [124-129] describing five relevant RCTs [130-135] (as no observational studies were found by our SR, we decided to use RCTs). These were then used to assess relative risk in this group of drugs relative to risks estimated based on primary, observational studies.
We used New Castle Ottawa Scale [20] for case-control and cohort studies to assess the quality of included studies. Observational studies of other types were assessed with focusing in methods of patients selection, methods of outcome recording (regarding only severe hypoglycaemia), study size and study representativeness. Overall studies’ quality varied. Among 9 case-control studies three scored 2 out of 9 possible points, three – 3 points, two – 4 points, and one – 5 points. Among 13 cohort studies one study scored 5 out of 9 possible points, six – 6 points, and six – 7 points. The residual studies, assessed by description with no scoring, were of medium quality. Details on quality of included studies is given in Online Resources ESM_5.
Systematic review of secondary studies
Literature search for other antidiabetic drugs yielded 12 systematic reviews (see fig. 2 in ESM_6), from which a study conducted by Karagiannis et al. [136] was assessed to provide the most appropriate data on severe hypoglycaemia associated with various antidiabetic medications in type 2 diabetes (for reference list of included studies and excluded studies with justification see ESM_7).
Data from the RCTs included in the study Karagiannis [136] indicated that in insulin-naïve patients with T2 DM treatment with sulfonylureas resulted in higher SHEs rate than treatment with other OADs (0.009 vs 0.0008 events per person-year, respectively, in patients treated with SU and patients treated with OADs other than SU – the estimated relative rate was 14.14, 95% CI: 5.53; 47.18, for the comparison of SU to DPP-4, while there was no statistical proof to differentiate the risk rate between these other OADs. The fact that SUs are related with greatest risk among all the OADs supports the approach to concentrate on SU risk in the systematic review of primary studies.
SHEs rates for various treatments
Systematic review carried out for SHEs risk in assumed drug groups provided data on absolute annual number of SHEs per one treated patient with diabetes. Results available in each of the studies split by diabetes type are presented in Tables 1 and 2. The quantitative analysis of these data resulted in the following mean annual SHEs rates per person are presented in Table 3.
Our results show that SHEs rates differ among drug regimens. In T1 DM basal-bolus insulin therapy with human insulin as the basal component was associated with the highest risk of SHEs (1.1 events per person-year) while the insulin pumps led to the lowest risk of SHEs (0.18 events per person-year). In type 2 diabetes basal-bolus insulin therapy with basal human insulin was also associated with the highest risk of SHEs (0.56 events per person-year) and patients may be at the lowest rate of SHEs when treated with OADs other than SU (0.006 events per patient-year). This pattern in type 2 diabetes may reflect the disease progression, from oral antidiabetic medications to insulin in monotherapy or combined with OADs.
Table 1. Diabetes type I results of the included studies
| Study | Time horizon (years) | Number of participants | Patients with ≥1 SHE | Number of events | Mean events no per patient-year | SD |
| Insulin pump therapy | ||||||
| Bruttomesso 2002 | 7.4 | 138 | 92 | 0.09 | 0.02 | |
| de Bock 2012 | 3 | 75 | 11 | 5 | ||
| Garg 2004c | 0.97 | 216 | 45 | 84 | 0.4 | Not clear |
| Jakisch 2008 | 1 | 412/300/199 | 74/60/34 | 17.87/20.04/17.33 | 2.85/3.91/4.47 | |
| Kapellen 2007 | 1/1/2.75/2.75 | 248/544/76/177 | 0.25/0.14/0.27/0.27 | |||
| Katz 2012 | 1.69 | 93 | 50 | 31.8 | ||
| Leinung 2010 | 1 | 117 | 37 | 68 | 58.9 | |
| Muller-Godeffroy 2009 | 0.5 | 88 | 6 | |||
| Nimri 2006 | 1/1/1 | 127/129/23 | 11.1/23.3/0 | |||
| Reda 2007 | 2.6 | 105 | 15 | 0.05 | ||
| Rudolph 2002 | 3.01 | 107 | 19.2 | |||
| Scaramuzza 2011 | 1.7/1.4 | 493/493 | 6.6/3.9 | |||
| Scheidegger 2007 | 0.46 | 19 | 1 | 1 | ||
| Wood 2006 | 1 | 132 | 7.4 | |||
| Basal bolus with long-acting insulin analogue | ||||||
| DAFNE, Keen 2012 | 1/1 | 124/124 | 15/6 | 37/22 | ||
| Garg 2004 a | 1.09 | 292 (98, 299) | 81 (28, 81) | 167 (n.a., n.a.) | 0.57 (0.5, 0.6) | |
| Herwig 2007 | 1.68 | 74 | 11 | 0.14 | 0.4 | |
| Kapellen 2009 | 1 | 6558 | 32.2/100 | 3 | ||
| Katz 2012 | 1.8 | 50 | 31 | 34.4 | ||
| Kristensen 2012 | 1 | 1052 | 1.47 | SE=0.18 | ||
| PREDICTIVE, Marre 2009 | 1 | 647 | 11 | 0.02 | ||
| PREDICTIVE, Preumont 2009 | 0.5 | 232 | 0.1 | 0.7 | ||
| PREDICTIVE, Sreenan 2008 | 0.23 | 1500 | 0.52 | |||
| PREDICTIVE, Yenigun 2009 | 0.08/0.23 | 506/506 | 94/28 | |||
| Basal bolus with human basal insulin | ||||||
| Garg 2004b | 1.06 | 98 | 30 | 1.2 | SEM=0.40 | |
| Hartemann-Heurtier 2003 | 1/1 | 110/110 | 14/26 | 0.2/0.83 | 0.62/3/34 | |
| Herwig 2007 | 1.68 | 68 | 62 | 0.73 | 1.68 | |
| Kristensen 2012 | 1 | 2085 | 1.09 | SE=0.11 | ||
| Leckie 2005 | 1 | 243 | 83 | 0.98 | ||
| PREDICTIVE, Sreenan 2008 | 0.077 | 1500 | 3.51 | |||
Table 2. Diabetes type II results of the included studies
| Study | Time horizon (years) | Number of participants | Patients with ≥1 SHE | No of events – absolute or mean per patient-year |
| Basal long-acting insulin analogue ± OADs | ||||
| A1chieve, Home 2011 | 0.46 | 12 078 and 3467 | 0 and 0.01 | |
| EARLY, Hanefeld 2012 | 0.46 | 1389 | 1 | 1 |
| FINE, Tsai 2011 | 0.50 | 2016 and 16 | 0.003 and 0 | |
| IMPROVE, Gumprecht 2009 | 0.25 | 245 | 0.197 | |
| Kawamori 2008 | 0.46 | 97 | 0 | 0 |
| LIGHT, Verges 2012 | 0.25 | 1863 | 18 | 0.12 |
| PREDICTIVE, Dornhorst 2008 b | 0.08 | 118 | 0.26 | |
| PREDICTIVE, Meneghini 2009 | 0.23 | 1652 | 0.00 | |
| PRESENT, Jang 2008 | 0.23 | 348 | 1.1 | |
| Sudhakaran 2010 | 0.46 | 54 | 0 | 0 |
| Sudhakaran 2011 | 0.46 | 2743 | 0 | 0 |
| Yang 2012 | 0.31 | 297 | 2 | 2 |
| Basal human insulin ± OADs | ||||
| FINE, Tsai 2011 | 0.50 | 589 | 0.031 | |
| Furlong 2002 | 2.42 (median) | 133 and 67 | 6 and 1 | |
| Honkasalo 2010, Honkasalo 2011 | 1 | 431 | 53 (12.3%) | 116 |
| IMPROVE, Gumprecht 2009 | 0.25 | 497 | 0.153 | |
| PREDICTIVE, Dornhorst 2008 b | 0.08 | 175 | 0.78 | |
| PRESENT, Jang 2008 | 0.23 | 3414 | 0.39 | |
| Sudhakaran 2010 | 0.46 | 23 | 0 | 0 |
| Basal bolus with long-acting insulin analogue ± OADs | ||||
| A1chieve, Home 2011 | 0.46 | 1593 and 2512 | 0 and 0.001 | |
| JDDM23, Oishi 2012 | 0.50 | 126 | 1 | 1 |
| PREDICTIVE, Sreenan 2008 | 0.23 | 2137 | 0 | |
| SAFIR, Zick 2007 | 0.15 | 455 | 0.7% of patients | 0.05 |
| Suzuki 2012 | 1 | 400 | 1 | 1 |
| Zjačić-Rotkvić 2012 | 0.5 | 203 | 0 | 0 |
| Basal bolus with human insulin ± OADs | ||||
| Biesenbach 2006 | 1 | 34 | 0.05 | |
| JDDM23, Oishi 2012 | 0.23 | 126 | 1 | 1 |
| PREDICTIVE, Sreenan 2008 | 008 | 2137 | 0.78 per patient year | |
| Pre-mix insulin analogues | ||||
| A1chieve, Home 2011 | 0.46 | 27 591 and 13 318 | 0 and 0.20 per patient-year | |
| BIAsp Start, Berntorp 2011 | 0.52 | 1154 | 2 | 2 |
| Danish BIAsp Study Group, Breum 2008 | 0.5 | 392 | 4 | |
| IMPROVE, Khader 2010 | 0.5 | 1613 | 0.05 | |
| IMPROVE, Valensi 2009 | 0.5 | 52 419 | 0.008 | |
| INITIATE plus, Oyer 2011 | 0.46 | 4812 | 87 | 127 |
| Levit 2011 | 2.9 | 115 | 0 | 0 |
| Ligthelm 2009 | 1.5 | 149 | 0 | 0 |
| Makela 2012 | 0.5 | 496 | 19 | |
| Nobels 2012 | 0.5 | 498 | 6 | |
| PRESENT, Gao 2009 | 0.23 | 3697; 4754; 2392; 817 | 0.04; 0.13; 0.3; NA | |
| PRESENT, Khutsoane 2008 | 0.50 | 21 977 | 0.1 | |
| Temizel 2010 | 1 | 71 | 0.06 per patient- month | |
| The 1-2-3 study, Garber 2006 | 0.31 | 100 and 68 and 25 | 3 and 3 and 1 | |
| Pre-mix human insulin | ||||
| Gu 2012 | 0.31 and 0.31 | 409 and 235 | 2 and 0 | |
| IMPROVE, Shah 2009 a | 0.25 | 3856 | 0.355 | |
| Nobels 2012 | 0.08 | 592 | 4 | |
| PRESENT, Shestakova 2007 | 0.23 | 3241 | 162 | 0.7 |
| Progens-first-step, Strojek 2008 | 0.25 and 0.25 | 482 and 483 | 1 and 2 patients during first 13-week observation and during second 13 weeks, respectively | 2 and 2 episodes, respectively |
| Temizel 2010 | 1 | 69 | 0.04 per patient-month | |
| SU | ||||
| Andayani 2010 | 0.5 | 49 | 1 | 1 |
| Aung 2012 | 1 | 10.43 | 24 | |
| Exhype, Pettersson 2011 | 0.5 | 430 | 5 (1.2%) | |
| Iványi 2012 | 2.54 | 86 | 2 | 2 |
| UK Hypoglycaemia Study Group | 0.73 | 103 | 0.1 | |
| Vexiau 2008 | 0.5 | 400 | 16 | |
Table 3. Annual mean (95% CI) number of SHEs in patients with type 1 and type 2 DM
| Therapy | Average number of SHEs per patient per year | 95% CI | Remarks |
| Type 1 DM | |||
| basal-bolus (basal insulin analogue) | 0.53 | 0.29–1.18 | |
| basal-bolus (basal human insulin) | 1.10 | 0.57–2.71 | |
| insulin pump | 0.18 | 0.13–0.25 | |
| pre-mix insulin analogue and pre-mix human insulin | 1.10 | due to lack of statistically significant differences between pre-mix human insulin and pre-mix insulin analogues, the same SHEs rate as for pre-mixed insulin analogues (so BBH) | |
| Type 2 DM | |||
| BOT analogue | 0.13 | 0.04–1.17 | |
| BOT human | 0.21 | 0.08–0.88 | |
| basal-bolus (basal insulin analogue) | 0.01 | 0.003–0.25 | |
| basal-bolus (basal human insulin) | 0.56 | 0.16–9.65 | |
| pre-mix insulin analogue | 0.10 | 0.05–0.26 | |
| pre-mix human insulin | 0.20 | 0.07–0.93 | |
| sulfonylureas | 0.05 | 0.02–0.14 | |
| OADs (excl. SU) | 0.006 | 0.001–0.008 |
Discussion
We conducted a systematic review and meta-analysis in order to estimate average annual rates of severe hypoglycaemia events associated with various insulin regimens and other antidiabetic medications. For insulin therapy and sulphonylureas we included observational studies that met the predefined criteria to directly assess rates of SHEs. For residual antidiabetic medications in type 2 diabetes we used data from another systematic review to assess the relative SHE frequency and apply it to a baseline rate estimated for SU. Due to lack of observational data for premix therapies for type 1 we had to refer to secondary studies as well in order to assess the relative risks in comparison to other therapies and indirectly calculate associated SHEs rates. That is why this part of results should be treated with greater caution.
The inclusion criteria for observational studies were defined so as to obtain as high quality of identified studies as possible. Thus, we decided to use newer publications only to account in possible changes of diabetes management over time (only studies published from 2002 on were used). Further we took into account only studies with at least 100 participants (we did not want to include small studies of a poor quality as the number of participants is also assessed in The Newcastle-Ottawa Scale). Most importantly the definition of SHE used in the identified studies was carefully checked so as to guarantee consistency among them, but at the same time we had to reject numerous studies due to lack of information in the definition used therein. That reduces the body of evidence but provides greater consistency of results. The overall quality of the studies, as measured by the New Castle Ottawa scale, is nonetheless rather medium. The most frequent shortcomings of the included case control studies were no definition of controls and using self reports or medical records only for the ascertainment of exposure. Major shortcoming of the included cohort studies was that it was not demonstrated that the outcome of interest was not presented at the start of the study. The heterogeneity of the studies is quite substantial, that is why a random effects model was used, and the resulting confidence intervals for mean rates are rather wide. We still have to notice that best available evidence was used, and so these limitations simply suggest the direction for further research when more observational studies have been published. With more data a better assessment of overall means should be possible, and perhaps a meta-regression approach could explain some sources of heterogeneity.
The applied methodology allowed to use two types of results reported in the studies, either number of SHEs or fraction of patients with at least one episode. As can be seen in tables 1 and 2, various reporting was used in identified observational studies. Focusing on number of SHEs only would substantially reduce the amount of data available, and that is why we decided to assume the Poisson distribution. Obviously, this assumption comes at a price, as potential biases may emerge. Poisson distribution forces the mean being equal to the variance, while hypoglycaemia events may concentrate in single patients more than this distribution would suggest (e.g. patient lifestyle either diminishes or augments chances of an event), but may also spread out more evenly (e.g. a patient having experience SHE will adapt her lifestyle to reduce future risk). We considered using another distribution (e.g. negative binomial) to allow for difference between mean and variance, but additional parameters made the estimation process and results very unstable. Secondly, it was mostly in T2 that substantial amount of data came in the form of number of patients with at least one SHE, where the overall risk was quite small and so the discrepancy between Poisson and some other distribution would be much smaller.
Eventually, annual rates varied from 0.18 for insulin pump up to 1.1 for basal-bolus with human basal insulin and from 0.006 for oral antidiabetic drugs up to 0.21 for basal human insulin with oral antidiabetic medications for type 1 and type 2 DM, respectively. Spread of results between individual studies is large, which means that several other factors may affect the outcome (e.g. life style). More data would probably make it possible to identify these factors, e.g. by meta-regression. However, the mean value may be still estimated and our calculations are based on the best (available at the time of the review) data.
It is worth to mention that our analysis of observational studies yielded results different from those based on RCTs. And so the risk of SHE associated with sulfonylureas estimated from observational studies amounted to 0.05 event per patient per year, and was higher than 0.01 coming from RCTs included in the systematic review by Karagiannis et al. [136]. This can lead to the conclusion that a real SHEs risks are higher than those in RCTs due to factors other than therapy associated with hypoglycaemia occurrence, however obviously both numbers are estimated with an error, and both are actually small in absolute terms.
It’s important to notice that our purpose was not to compare given drugs between themselves – that is we defined our approach so as to get the best possible estimate of SHE rate for each treatment separately, rather than the best possible estimate of relative SHE rate between pairs of treatment regimes. The latter would require e.g. looking for studies with several arms encompassing more than one treatment regimen, so as to get relative effects and then meta-analyse them (while we meta-analysed individual treatment rates for each regimen separately). Another important decision would then also be whether to use interventional or observational studies, and that depends largely on a question we are asking. If we wanted to know – “what is the risk if I give this treatment to my patient?” – we should rather go for interventional studies. In our case our question rather is – “what is the risk if I observe this patient using this treatment” – and then observational studies seem to be more appropriate, as they account for the fact that some patients may be using drugs that address their life-style and moderates their baseline SHE risk. Additionally, observational studies do not impose very strict protocol that may bias complication rates downwards in RCTs when compared to real-life situations. Thus, our results should not be used to quantify consequences of switching patients between drug regimens, but rather to assess the actual overall burden of SHE when drug usage patterns are known.
We did not find other systematic review or meta-analysis that evaluate real-life risk of severe hypoglycaemia among diabetic patients (type 1 and type 2) for various therapies. A review closest to ours was the one conducted by Bolen et al. [137] that summarized the English-language literature on the benefits and harms of oral agents in adult patients with T2 DM. In their review, Bolen et al. included 216 controlled trials and cohort studies and 2 systematic reviews in total of which 169 articles evaluated adverse events. In comparison to our review they estimated weighted absolute risk differences between individual drugs, drug groups or therapies, while our aim was to estimate average annual rates of SHEs associated with various insulin regimens and other antidiabetic medications. Moreover, they presented combined results for minor and major hypoglycaemia and did not provide the definition of major hypoglycaemia. Results of their meta-analysis indicated that in patients receiving second generation sulfonylureas hypoglycaemic episodes (minor and major) were more frequent than in patients receiving metformin or TZD. They obtained concordant conclusion as can be seen in NICE, IDF, ADA and EASD guidelines [14-17] in the treatment of type 2 DM which indicate that sulfonylureas are associated with higher risk of hypoglycaemia than other antidiabetic oral drugs.
Other meta-analysis of observational studies conducted in patients with T2 DM by Goto et al. [138] evaluated association between severe hypoglycaemia and risk of cardiovascular disease. Cohort studies and randomised controlled trials were included as long as an observational analysis of the analysed association was available. Goto et al. included six studies in their meta-analysis (two were secondary analyses of RCT and four were based on administrative databases) of which none fulfilled inclusion criteria of our systematic review due to inappropriate definition of severe hypoglycaemia. The association between SHE and cardiovascular disease was estimated with the use of relative risk as a measure of effect. Results suggest that severe hypoglycaemia is associated with approximately twice the risk of cardiovascular disease. These results indicate the need for evaluation and quantification of the risk of severe hypoglycaemia.
Conclusions
Various drug regimens differ in terms of severe hypoglycaemia risk, as also pointed out in published guidelines. Our results indicate that basal-bolus therapy with basal human insulin is associated with the highest average number of SHEs per patient per year, both in type 1 and type 2 DM, while insulin pump and OADs (excl. SU) seems to be the safest therapies in T1 and T2 diabetes, respectively. These differences can be quantified based on results of published observational studies. Results of the current analysis can be used to provide parameters for cost-of-illness studies estimating the overall burden of hypoglycaemia.
List of abbreviations
AD – antidiabetic medication
BB – basal-bolus
BBA – basal-bolus insulin therapy with long-acting insulin analogue as the basal component
BBH – basal-bolus insulin therapy with human insulin as the basal component,
BOTA – basal supported oral therapy with long-acting insulin analogue as the basal component
BOTH – basal supported oral therapy with human insulin as the basal component
CI – confidence interval
DM – diabetes mellitus
OAD – oral antidiabetic medication
RCT – randomized controlled trial
SHE – severe hypoglycaemia event
SU – sulfonylurea
T1, T2 DM – type 1, type 2 diabetes mellitus
TZD – thiazolidinediones
Competing interests
The project was funded by Novo Nordisk. The author(s) declare that they have no competing interests. There is no specific organization that may in any way gain or lose financially from the publication of this manuscript.
Authors’ contributions
MJ, JP, MN and MC are the authors of general analytic framework. MJ, JP, ER and MN have participated in the systematic review. All authors participated in preparing, read and approved the final manuscript.
Acknowledgments
The authors would like to acknowledge the following people: Jelka Zaletel, Tomáš Doležal, Bence Nagy, Tereza Šarić, Karel Rychna, Irina Ryzhenkova, Sanda Sandalj, and Zsofia Tarjanyi for their helpful comments. Acknowledgments also go to Novo Nordisk, a sponsor of this project.
- Ahrén B.: Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Manag 2013, 9:155-63
- Saunders R., Lian J., Karolicki B., Valentine W.: The cost-effectiveness and budget impact of stepwise addition of bolus insulin in the treatment of type 2 diabetes: evaluation of the FullSTEP trial. J Med Econ, in press
- Brown ST., Grima DG., Sauriol L.: Cost-Effectiveness of Insulin Glargine Versus Sitagliptin in Insulin-Naïve Patients With Type 2 Diabetes Mellitus..Clin Ther, in press
- Evans M., Wolden M., Gundgaard J., Chubb B., Christensen T.: Cost-effectiveness of insulin degludec compared with insulin glargine in a basal-bolus regimen in patients with type 1 diabetes mellitus in the UK. J Med Econ, in press
- Kiadaliri AA., Gerdtham UG., Eliasson B., Carlsson KS.: Cost-Utility Analysis of Glucagon-Like Peptide-1 Agonists Compared with Dipeptidyl Peptidase-4 Inhibitors or Neutral Protamine Hagedorn Basal Insulin as Add-On to Metformin in Type 2 Diabetes in Sweden. Diabetes Ther, in press
- Ly TT., Brnabic AJ., Eggleston A., Kolivos A., McBride ME., Schrover R., Jones TW.: A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health 2014, 17:561-569
- Jönsson L., Bolinder B., Lundkvist J.: Cost of hypoglycemia in patients with Type 2 diabetes in Sweden. Value Health 2006, 9:193–198
- Holstein A., Plaschke A., Egberts EH.: Incidence and costs of severe hypoglycemia. Diabetes Care 2002, 25:2109–2110
- Leese GP., Wang .J, Broomhall J., Kelly P., Marsden A., Morrison W., Frier BM., Morris AD.; DARTS/MEMO Collaboration: Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care 2003, 26:1176–1180
- Miller CD., Phillips LS., Ziemer DC., Gallina DL., Cook CB., El-Kebbi IM.: Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med 2001, 161:1653–1659
- UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352:837–353
- van Staa T., Abenhaim L., Monette J.: Rates of hypoglycemia in users of sulfonylureas. J Clin Epidemiol 1997, 50:735–741
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses; Available from: http://www.prisma-statement.org/. [Accessed: 8.10.2014.]
- International Diabetes Federation: Global Guideline for Type 2 Diabetes; Available from: http://www.idf.org/global-guideline-type-2-diabetes-2012
- Inzucchi SE., Bergenstal RM., Buse JB., Diamant M., Ferrannini E., Nauck M., Peters AL., Tsapas A., Wender R., Matthews DR.: Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35:1364–1379
- National Institute for Health and Clinical Excellence. Short Clinical Guideline 87.: Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes; Available from: http://guidance.nice.org.uk
- National Institute for Health and Clinical Excellence. Clinical Guideline 15.: Type 1 diabetes: diagnosis and management of type 1 diabetes in children, young people and adults; Available from:http://guidance.nice.org.uk
- Morales J.: The pharmacologic basis for clinical differences among GLP-1 receptor agonists and DPP-4 inhibitors. Postgrad Med 2011, 123:189-201
- Reid T.: Choosing GLP-1 Receptor Agonists or DPP-4 Inhibitors: Weighing the Clinical Trial Evidence.Clin Diabetes 2012, 30:3-12
- The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Deeks JK., Dinnes J., D’Amico R., Sowden AJ., Sakarovitch C., Song F., Petticrew M., Altman DG.; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group: Evaluating non-randomised intervention studies. Health Technol Assess 2003, 7:1-192
- Higgins JPT., Green S.: Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Chapter 13.5.2.3 (Tools for assessing methodological quality or risk of bias in non-randomized studies); Available from: http://handbook.cochrane.org/chapter_13/13_5_2_3_tools_for_assessing_methodological_quality_or_risk_of.htm
- Ali M., White J., Lee CH., Palmer JL., Smith-Palmer J., Fakhoury W., Valentine WJ.: Therapy conversion to biphasic insulin aspart 30 improves long-term outcomes and reduces the costs of type 2 diabetes in Saudi Arabia. J Med Econ 2008, 11:651-670
- Almustafa M., Yeo JP., Khutsoane D.: Glycaemic control and hypoglycaemia in the PRESENT study. Diabetes Res Clin Pract 2008, 81(Suppl 1): S10-S15
- Andayani TM., Ibrahim MIM., Asdie AH.: The safety of triple therapy with oral antidiabetics versus insulin in type 2 diabetes. Asian J Pharm Clin Res 2010, 3: 201-203
- Aung PP., Strachan MWJ., Frier BM., Butcher I., Deary IJ., Price JF.; Edinburgh Type 2 Diabetes Study Investigators: Severe hypoglycaemia and late-life cognitive ability in older people with Type 2 diabetes: The Edinburgh Type 2 Diabetes Study. Diabetic Med 2012, 29: 328-336
- Berntorp K., Haglund M., Larsen S., Petruckevitch A., Landin-Olsson M.; Swedish BIAsp Study Group: Initiation of biphasic insulin aspart 30/70 in subjects with type 2 diabetes mellitus in a largely primary care-based setting in Sweden. Prim Care Diabetes 2011, 5: 89-94
- Biesenbach G., Bodlaj G., Pieringer H.: Weight gain and metabolic control in newly insulin-treated patients with type 2 diabetes with different insulin regimens. Can J Diabetes 2006, 30: 384-389
- Breum L., Almdal T., Eiken P., Lund P., Christiansen E., on behalf of the Danish BIAsp Study Group: Initiating or switching to biphasic insulin aspart 30/70 therapy in subjects with type 2 diabetes mellitus. An observational study. Rev Diabet Stud 2008, 5:154-162
- Brod M., Valensi P., Shaban JA., Bushnell DM, Christensen TL: Patient treatment satisfaction after switching to NovoMix(R) 30 (BIAsp 30) in the IMPROVE study: an analysis of the influence of prior and current treatment factors. Qual Life Res 2010, 19:1285-1293
- Bruttomesso D., Pianta A., Crazzolara D., Scaldaferri E., Lora L., Guarneri G., Mongillo A., Gennaro R., Miola M., Moretti M., Confortin L., Beltramello GP., Pais M., Baritussio A., Casiglia E., Tiengo A.: Continuous subcutaneous insulin infusion (CSII) in the Veneto region: efficacy, acceptability and quality of life. Diabet Med 2002, 19: 628-634
- de Bock M., Gunn AJ., Holt JA., Derraik JG., Reed P., Cutfield W., Mouat F., Hofman P., Jefferies C.: Impact of insulin pumps on glycaemic control in a pump-naive paediatric regional population. J Paediatr Child Health 2012, 48: 247-252
- Dornhorst A., Lüddeke HJ., Honka M., Ackermann RW., Meriläinen M., Gallwitz B., Sreenan S.; PREDICTIVE Study Group: Safety and efficacy of insulin detemir basal-bolus therapy in type 1 diabetes patients: 14-Week data from the European cohort of the PREDICTIVE study. Curr Med Res Opin 2008, 24:369-376
- Dornhorst A., Lüddeke HJ., Koenen C, Meriläinen M., King A., Robinson A., Sreenan S.; PREDICTIVE Study Group: Transferring to insulin detemir from NPH insulin or insulin glargine in type 2 diabetes patients on basal-only therapy with oral antidiabetic drugs improves glycaemic control and reduces weight gain and risk of hypoglycaemia: 14-week follow-up data from PREDICTIVE®. Diabetes Obes Metab 2008, 10:75-81
- Dornhorst A., Lüddeke HJ., Sreenan S., Kozlovski P., Hansen JB., Looij BJ., Meneghini L.; PREDICTIVE Study Group: Insulin detemir improves glycaemic control without weight gain in insulin-naive patients with type 2 diabetes: Subgroup analysis from the PREDICTIVE® study. Int J Clin Pract 2008, 62:659-665
- Dornhorst A., Lüddeke HJ., Sreenan S., Koenen C., Hansen JB., Tsur A., Landstedt-Hallin L.: Safety and efficacy of insulin detemir in clinical practice: 14-Week follow-up data from type 1 and type 2 diabetes patients in the PREDICTIVETM European cohort. Int J Clin Pract 2007, 61: 523-528
- Esteghamati A., Rajabian R., Amini M., Bahrami A., Khamseh ME., Afkhami-Ardekani M., Rizi EP.: The safety and efficacy of biphasic insulin aspart 30 (BIAsp 30) in Iranians with type 2 diabetes: An open-label, non-randomised, multi-centre observational study - The Iran subgroup of the IMPROVE® study. Endokrynol Pol 2010, 61: 364-370
- Fontaine P., Gin H., Pinget M., Thivolet C., Hanaire H., Robert JJ., Marre M., Venkatanarasimhachar S.: Effect of insulin detemir dose frequency on clinical outcomes in patients with diabetes in PREDICTIVE. Adv Ther 2009, 26: 535-551
- Furlong NJ., McNulty SJ., O'Brien SV., Hardy KJ.: Comparison of metformin versus sulphonylurea in combination with daily NPH insulin in patients with type 2 diabetes inadequately controlled on oral hypoglycaemic agents; median follow-up 29 months. Practical Diabetes Int 2002, 19:245-249
- Gao Y., Guo XH., Vaz JA.; PRESENT Study Group: Biphasic insulin aspart 30 treatment improves glycaemic control in patients with type 2 diabetes in a clinical practice setting: Chinese PRESENT study. Diabetes Obes Metab 2009, 11:33-40
- Gao Y., Guo XH.: Switching from human insulin to biphasic insulin aspart 30 treatment gets more patients with type 2 diabetes to reach target glycosylated hemoglobin <7%: The results from the China cohort of the PRESENT study. Chin Med J (Engl) 2010, 123:1107-1111
- Garber AJ., Wahlen J., Wahl T., Bressler P., Braceras R., Allen E., Jain R.: Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab 2006, 8: 58-66
- Garg SK., Gottlieb PA., Hisatomi ME., D'Souza A., Walker AJ, Izuora KE., Chase HP.: Improved glycemic control without an increase in severe hypoglycemic episodes in intensively treated patients with type 1 diabetes receiving morning, evening, or split dose insulin glargine. Diabetes Res Clin Pract 2004, 66:49-56
- Garg SK., Paul JM., Karsten JI., Menditto L., Gottlieb PA.: Reduced severe hypoglycaemia with insulin glargine in intensively treated adults with type 1 diabetes. Diabetes Technol Ther 2004, 6:589-595
- Garg SK., Walker AJ., Hoff HK., D'Souza AO., Gottlieb PA., Chase HP.: Glycemic Parameters with Multiple Daily Injections Using Insulin Glargine Versus Insulin Pump. Diabetes Technol Ther 2004, 6:9-15
- Giorda C., Boemi M., Borzì V., Chiaramonte F., Mattei P., Tribulato A.: The IMPROVE study a multinational, multicentre, observational study in type 2 diabetes: results from the Italian cohort. Acta Biomed 2010, 81:115-124
- Gu Y., Hou X., Zhang L., Pan J., Cai Q., Bao Y., Jia W.: The impact of initiating biphasic human insulin 30 therapy in type 2 diabetes patients after failure of oral antidiabetes drugs. Diabetes Technol Ther 2012, 14:244-250
- Güler S., Sharma SK., Almustafa M., Kim CH., Azar S., Danciulescu R., Shestakova M., Khutsoane D., Bech OM.: Improved glycaemic control with biphasic insulin aspart 30 in type 2 diabetes patients failing oral antidiabetic Drugs: PRESENT study results.Arch Drug Inf 2009, 2:23-33
- Gumprecht J, Benroubi M, Borzi V, Kawamori R, Shaban J, Shah S, Shestakova M, Wenying Y, Ligthelm R, Valensi P; IMPROVE Study Group Expert Panel: Intensification to biphasic insulin aspart 30/70 (BIAsp 30, NovoMix® 30) can improve glycaemic control in patients treated with basal insulins: A subgroup analysis of the IMPROVE observational study.Int J Clin Pract 2009, 63:966-972
- Gumprecht J, Zurawska G, Wolnik B, Dzida G: The IMPROVE study - A multinational, observational study in type 2 diabetes: Data from the Polish cohort.Pol J Endocrinol 2008, 59:460-466
- Hanefeld M, Fleischmann H, Landgraf W, Pistrosch F. EARLY study:Early basal insulin therapy under real-life conditions in type 2 diabetics.Diabetes Stoffwech H 2012, 21:91-97
- Hartemann-Heurtier A, Sachon C, Masseboeuf N, Corset E, Grimaldi A: Functional intensified insulin therapy with short-acting insulin analog: effects on HbA1c and frequency of severe hypoglycaemia. An observational cohort study. Diabetes Metab 2003, 29:53-57
- Hassan MI., Aamir AH., Miyan Z., Siddiqui LA., Qureshi MS., Shaikh MZ.: Safety and effectiveness of biphasic insulin aspart 30 (BIAsp 30) in people with type 2 diabetes mellitus in the Pakistani population: Results from the A1chieve study. J Pak Med Assoc 2012, 62:929-936
- UK Hypoglycaemia Study Group: Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007, 50:1140-1147
- Hermansen K., Dornhorst A., Sreenan S.: Observational, open-label study of type 1 and type 2 diabetes patients switching from human insulin to insulin analogue basal-bolus regimens: insights from the PREDICTIVE study. Curr Med Res Opin 2009, 25:2601-2608
- Hermansen K., Lund P., Clemmensen K., Breum L., Kleis Moller M., Mette Rosenfalck A., Christiansen E.; Danish PREDICTIVE study group: 3-Month results from Denmark within the globally prospective and observational study to evaluate insulin detemir treatment in type 1 and type 2 diabetes: The PREDICTIVE study. Rev Diabet Stud 2007, 4:89-97
- Herwig J., Scholl-Schilling G., Böhles H.: Glycaemic control and hypoglycaemia in children, adolescents and young adults with unstable type 1 diabetes mellitus treated with insulin glargine or intermediate-acting insulin. J Pediatr Endocrinol Metab 2007, 20:517-525
- Home P., Naggar NE., Khamseh M., Gonzalez-Galvez G., Shen C., Chakkarwar P., Wenying Y.: An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract 2011, 94:352-363
- Honkasalo M., Elonheimo O., Sane T.: Many diabetic patients with recurrent severe hypoglycaemias hold a valid driving license. A community-based study in insulin-treated patients with diabetes.Traffic Inj Prev 2010, 11:258-262
- Honkasalo MT., Elonheimo OM., Sane T.: Severe hypoglycaemia in drug-treated diabetic patients needs attention: a population-based study. Scand J Prim Health Care 2011, 29:165-170
- Ishii H., Iwase M., Seino H., Shuto Y., Atsumi Y.: Assessment of quality of life in patients with type 2 diabetes mellitus before and after starting biphasic insulin aspart 30 (BIAsp 30) therapy: IMPROVE study in Japan. Curr Med Res Opin 2011, 27:643-650
- Iványi T., Fövényi J., Faludi P., Han J., Macconell L., Wille S., Kiljanski J.: Long-Term Effects of Adding Exenatide to a Regimen of Metformin and/or Sulfonylurea in Type 2 Diabetes: An Uncontrolled, Open-Label Trial in Hungary. Clin Ther 2012, 34:1301-1313
- Jakisch BI., Wagner VM., Heidtmann B., Lepler R., Holterhus PM., Kapellen TM., Vogel C., Rosenbauer J., Holl RW.; German/Austrian DPV Initiative and Working Group for Paediatric Pump Therapy: Comparison of continuous subcutaneous insulin infusion (CSII) and multiple daily injections (MDI) in paediatric Type 1 diabetes: a multicentre matched-pair cohort analysis over 3 years. Diabet Med 2008, 25:80-85
- Jang HC., Guler S., Shestakova M.; PRESENT Study Group: When glycaemic targets can no longer be achieved with basal insulin in type 2 diabetes, can simple intensification with a modern pre-mix insulin help? Results from a subanalysis of the PRESENT study. Int J Clin Pract 2008, 62:1013-1018
- Jang HC., Lee SR., Vaz JA.: Biphasic insulin aspart 30 in the treatment of elderly patients with type 2 diabetes: A subgroup analysis of the PRESENT Korea NovoMixstudy. Diabetes Obes Metab 2009, 11:20-26
- Kapellen TM., Heidtmann B., Bachmann J., Ziegler R., Grabert M., Holl RW.: Indications for insulin pump therapy in different age groups - An analysis of 1567 children and adolescents. Diabet Med 2007, 24:836-842
- Kapellen TM., Wolf J., Rosenbauer J., Stachow R., Ziegler R., Szczepanski R., Holl RW.; DPV-Science-Initiative: Changes in the use of analogue insulins in 37 206 children and adolescents with type 1 diabetes in 275 German and Austrian centres during the last twelve years. Exp Clin Endocrinol Diabetes 2009, 117:329-335
- Katz ML., Volkening LK., Anderson BJ., Laffel LM.: Contemporary rates of severe hypoglycaemia in youth with Type1 diabetes: Variability by insulin regimen. Diabet Med 2012, 29:926-932
- Kawamori R., Eliaschewitz FG., Takayama H., Hayashida CY.: Efficacy of insulin glargine and glimepiride in controlling blood glucose of ethnic Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2008, 79:97-102
- Kawamori R., Valensi P.: IMPROVE observational study of biphasic insulin aspart 30/70 in patients with Type 2 diabetes mellitus. Expert Rev Endocrinol Metab 2010, 5:507-516
- Keen AJ., Duncan E., McKillop-Smith A., Evans ND., Gold AE.: Dose Adjustment for Normal Eating (DAFNE) in routine clinical practice: who benefits? Diabet Med 2012, 29:670-676
- Khader S., Abdelfattah W., Almansari A., Elnnagar NK.: Safety and efficacy of switching to biphasic insulin aspart 30/70 (BIAsp 30) under the routine diabetic care in patients with type 2 diabetes: The IMPROVE observational study in the Gulf region. Int J Diabetes Mellit 2010, 2:110-113
- Khutsoane D., Sharma SK., Almustafa M., Jang HC., Azar ST., Danciulescu R., Shestakova M., Ayad NM., Guler S., Bech OM.; PRESENT Study Group: Biphasic insulin aspart 30 treatment improves glycaemic control in patients with type 2 diabetes in a clinical practice setting: experience from the PRESENT study. Diabetes Obes Metab 2008, 10:212-222
- Kristensen PL., Hansen LS., Jespersen MJ., Pedersen-Bjergaard U., Beck-Nielsen H., Christiansen JS., Nørgaard K., Perrild H., Parving HH., Thorsteinsson B., Tarnow L.: Insulin analogues and severe hypoglycaemia in type 1 diabetes.Diabetes Res Clin Pract 2012, 96:17-23
- Kurtoglu S., Atabek ME., Dizdarer C., Pirgon O., Isguven P., Emek S.; PREDICTIVE Turkey Study Group: Insulin detemir improves glycemic control and reduces hypoglycaemia in children with type 1 diabetes: Findings from the Turkish cohort of the PREDICTIVE® observational study. Pediatr Diabetes 2009, 10:401-407
- Leckie AM., Graham MK., Grant JB., Ritchie PJ., Frier BM..: Frequency, severity, and morbidity of hypoglycaemia occurring in the workplace in people with insulin-treated diabetes. Diabetes Care 2005, 28:1333-1338
- Leinung M., Thompson S., Nardacci E.: Benefits of continuous glucose monitor use in clinical practice. Endocr Pract 2010, 16:371-375
- Levit S., Toledano Y., Wainstein J.: Improved glycaemic control with reduced hypoglycaemic episodes and without weight gain using long-term modern pre-mix insulins in type 2 diabetes. Int J Clin Pract 2011, 65:165-171
- Ligthelm RJ.: Self-titration of biphasic insulin aspart 30/70 improves glycaemic control and allows easy intensification in a Dutch clinical practice. Prim Care Diabetes 2009, 3:97-102
- Luddeke HJ., Sreenan S., Aczel S., Maxeiner S., Yenigun M., Kozlovski P., Gydesen H., Dornhorst A.; PREDICTIVE Study Group: PREDICTIVE- a global, prospective observational study to evaluate insulin detemir treatment in types 1 and 2 diabetes: baseline characteristics and predictors of hypoglycaemia from the European cohort. Diabetes Obes Metab 2007, 9:428-434
- Makela JK., Schmuser C., Askonen K., Saukkonen T.: Starting or switching to biphasic insulin aspart 30 (BIAsp 30) in type 2 diabetes: a multicenter, observational, primary care study conducted in Finland. Diabetes Res Clin Pract 2012, 95:10-18
- Marre M., Pinget M., Gin H., Thivolet C., Hanaire H., Robert JJ., Fontaine P.: Insulin detemir improves glycaemic control with less hypoglycaemia and no weight gain: 52-week data from the PREDICTIVE study in a cohort of French patients with type 1 or type 2 diabetes. Diabetes Metab 2009, 35:469-475
- Meneghini LF., Dornhorst A., Sreenan S.; PREDICTIVE Study Group: Once-daily insulin detemir in a cohort of insulin-naive patients with type 2 diabetes: a sub-analysis from the PREDICTIVE study. Curr Med Res Opin 2009, 25:1029-1035
- Meneghini LF., Rosenberg KH., Koenen C., Merilainen MJ., Lüddeke HJ.: Insulin detemir improves glycaemic control with less hypoglycaemia and no weight gain in patients with type 2 diabetes who were insulin naive or treated with NPH or insulin glargine: clinical practice experience from a German subgroup of the PREDICTIVE study. Diabetes Obes Metab 2007, 9:418-427
- Müller-Godeffroy E., Treichel S., Wagner VM.; German Working Group for Paediatric Pump Therapy.: Investigation of quality of life and family burden issues during insulin pump therapy in children with Type 1 diabetes mellitus - Tempa large-scale multicentre pilot study. Diabet Med 2009, 26:493-501
- Nimri R., Weintrob N., Benzaquen H., Ofan R., Fayman G., Phillip M.: Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics 2006, 117:2126-2131
- Nobels F., D'Hooge D., Crenier L.: Switching to biphasic insulin aspart 30/50/70 from biphasic human insulin 30/50 in patients with type 2 diabetes in normal clinical practice: Observational study results. Curr Med Res Opin 2012, 28:1017-1026.
- Oishi M., Abe N., Yokoyama H., Kuribayashi N., Tomonaga O., Matoba K., Kobayashi M.; Japan Diabetes Clinical Data Management Study Group: Observational 6-month open-label study of Japanese type 2 diabetes patients switching from NPH insulin to insulin detemir in basal-bolus regimen: 23rd article of the Japan diabetes clinical data management study group (JDDM23).J Int Med Res 2012, 40:787-797
- Oyer DS., Shepherd MD., Coulter FC., Bhargava A., Deluzio AJ., Chu PL., Trippe BS.; Initiateplus Study Group: Efficacy and Tolerability of Self-Titrated Biphasic Insulin Aspart 70/30 in Patients Aged >65 Years With Type 2 Diabetes: An Exploratory Post Hoc Subanalysis of the INITIATEplus Trial. Clin Ther 2011, 33:874-883
- Peczyńska J., Urban M., Głowińska B., Florys B.: Decreased consciousness of hypoglycaemia and the incidence of severe hypoglycaemia in children and adolescents with diabetes type 1. EndokrynolDiabetol Chor Przemiany Materii Wieku Rozw 2002, 8:77-82
- Perriello G., Caputo S., De Pergola G., Di Carlo A., Grassi G., Lapolla A., Pata P., Solerte SB., Zaccardi F.: Improved glycemic control with weight loss and a low risk of hypoglycaemia with insulin detemir: insights from the Italian cohort of the PREDICTIVE study after 6-month observation in type 2 diabetic subjects. Expert Opin Pharmacother 2011, 12:2449-2455
- Pettersson B., Rosenqvist U., Deleskog A., Journath G., Wändell P.: Self-reported experience of hypoglycaemia among adults with type 2 diabetes mellitus (Exhype). Diabetes Res Clin Pract 2011, 92:19-25
- Preumont V., Buysschaert M., De Beukelaer S., Mathieu C.: Insulin detemir in routine clinical practice: A 26-week follow-up in type 1 diabetic patients from the Belgian PREDICTIVE cohort. Acta Clin Belg 2009, 64:49-55
- Reda E., Von Reitzenstein A., Dunn P.: Metabolic control with insulin pump therapy: the Waikato experience. N Z Med J 2007, 120:U2401
- Rudolph JW., Hirsch IB.: Assessment of therapy with continuous subcutaneous insulin infusion in an academic diabetes clinic. Endocr Pract 2002, 8:401-405
- Scaramuzza AE., Iafusco D., Rabbone I., Bonfanti R., Lombardo F., Schiaffini R., Buono P., Toni S., Cherubini V., Zuccotti GV.; Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetology: Use of integrated real-time continuous glucose monitoring/insulin pump system in children and adolescents with type 1 diabetes: a 3-year follow-up study. Diabetes Technol Ther 2011, 13:99-103
- Scheidegger U., Allemann S., Scheidegger K., Diem P.: Continuous subcutaneous insulin infusion therapy: effects on quality of life.S wiss Med Wkly 2007, 137:476-482
- Shah S., Benroubi M., Borzi V., Gumprecht J., Kawamori R., Shaban J., Shestakova M., Wenying Y., Valensi P.; IMPROVE Study Group Expert Panel.: Safety and effectiveness of biphasic insulin aspart 30/70 (NovoMix® 30) when switching from human premix insulin in patients with type 2 diabetes: Subgroup analysis from the 6-month IMPROVE observational study. Int J Clin Pract 2009, 63:574-582
- Shah S., Das AK., Kumar A., Unnikrishnan AG., Kalra S., Baruah MP., Ganapathi B., Sahay RK.: Baseline characteristics of the Indian cohort from the IMPROVE study: a multinational, observational study of biphasic insulin aspart 30 treatment for type 2 diabetes. Adv Ther 2009, 26:325-335
- Shah S., Zilov A., Malek R., Soewondo P., Bech O., Litwak L.: Improvements in quality of life associated with insulin analogue therapies in people with type 2 diabetes: Results from the A1chieve observational study. Diabetes Res Clin Pract 2011, 94:364-370
- Shah SN., Litwak L., Haddad J., Chakkarwar PN., Hajjaji I.: The A1chieve study: a 60 000-person, global, prospective, observational study of basal, meal-time, and biphasic insulin analogs in daily clinical practice. Diabetes Res Clin Pract 2010, 88(Suppl 1):S11-S16
- Sharma SK., Al-Mustafa M., Oh SJ., Azar ST., Shestakova M., Guler S., Vaz JA.: Biphasic insulin aspart 30 treatment in patients with type 2 diabetes poorly controlled on prior diabetes treatment: Results from the PRESENT study. Curr Med Res Opin 2008, 24:645-652
- Sharma SK., Joshi SR., Kumar A., Unnikrishnan AG., Hoskote SS., Moharana AK., Chakkarwar PN., Vaz JA.; PRESENT Study Group: Efficacy, safety and acceptability of biphasic insulin aspart 30 in Indian patients with type 2 diabetes: results from the PRESENT study. J Assoc Physicians India 2008, 56:859-863
- Shestakova M., Bech OM., Momani MS.: Study design and baseline characteristics of patients in the PRESENT study. Diabetes Res Clin Pract 2008, 81(Suppl 1):S3-S9
- Shestakova M., Sharma SK., Almustafa M., Min KW., Ayad N., Azar ST., Danciulescu R., Khutsoane D., Guler S.: Transferring type 2 diabetes patients with uncontrolled glycaemia from biphasic human insulin to biphasic insulin aspart 30: experiences from the PRESENT study. Curr Med Res Opin 2007, 23:3209-3214
- Sreenan S., Virkamaki A., Zhang K., Hansen JB.; PREDICTIVE study group: Switching from NPH insulin to once-daily insulin detemir in basal-bolus-treated patients with diabetes mellitus: Data from the European cohort of the PREDICTIVE® study. Int J Clin Pract 2008, 62:1971-1980
- Strojek K., Tarasiuk A., Bijos P., Czech A.: Gensulin M30 in patients with type 2 diabetes and secondary failure to oral antidiabetic drugs. the Progens-first-step study: A multicentre observational study in the outpatient setting. Diabet Dośw i Klin 2008, 8:179-184
- Sudhakaran C., Fathima M., Anjana RM., Unnikrishnan RI., Mohan V.: Effectiveness of exenatide in Asian Indians in a clinical care setting. Diabetes Technol Ther 2010, 12:613-618
- Sudhakaran C., Kishore U., Anjana RM., Unnikrishnan R., Mohan V.: Effectiveness of sitagliptin in asian Indian patients with type 2 diabetes-an Indian tertiary diabetes care center experience. Diabetes Technol Ther 2011, 13:27-32
- Suzuki D., Toyoda M., Kondo M., Miyatake H., Tanaka E., Sato H., Kuriyama Y., Miyauchi M., Yamamoto N., Kimura M., Umezono T., Fukagawa M.: Efficacy of long-acting insulin analog insulin glargine at high dosage for basal-bolus insulin therapy in patients with type 2 diabetes. Tokai J Exp Clin Med 2012, 37:35-40
- Temizel M., Mert M., Bozbey C., Arman Y., Cevizci E., Altintaş N., Cetin Ölek A.: Evaluation of the weight-increasing effects of biphasic analog and regular NPH insulin mixtures in patients with Type 2 diabetes mellitus. J Diabetes 2010, 2:250-255
- Tsai ST., Pathan F., Ji L., Yeung VT., Chadha M., Suastika K., Son HS., Tan KE., Benjasuratwong Y., Nguyen TK., Iqbal F.: First insulinization with basal insulin in patients with Type 2 diabetes in a real-world setting in Asia. J Diabetes 2011, 3:208-216
- Valensi P., Benroubi M., Borzi V., Gumprecht .J, Kawamori R., Shaban J., Shah S., Shestakova M., Wenying Y.; IMPROVE Study Group Expert Panel: Initiating insulin therapy with, or switching existing insulin therapy to, biphasic insulin aspart 30/70 (NovoMix 30) in routine care: Safety and effectiveness in patients with type 2 diabetes in the IMPROVE observational study. Int J Clin Pract 2009, 63:522-531
- Valensi P., Benroubi M., Borzi V., Gumprecht J., Kawamori R., Shaban J., Shah S., Shestakova M., Wenying Y.; IMPROVE Study Group Expert Panel: The IMPROVE study-a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract 2008, 62:1809-1819
- Vergès B., Brun JM., Tawil C., Alexandre B., Kerlan V.: Strategies for insulin initiation: insights from the French LIGHT observational study. Diabetes Metab Res Rev 2012, 28:97-105
- Vexiau P., Mavros P., Krishnarajah G., Lyu R., Yin D.: Hypoglycaemia in patients with type 2 diabetes treated with a combination of metformin and sulphonylurea therapy in France. Diabetes Obes Metab 2008, 10(Suppl 1):16-24
- Wenying Y., Benroubi M., Borzi V., Gumprecht J., Kawamori R., Shaban J., Shah S., Shestakova M., Ligthelm R., Valensi P.; IMPROVE Study Group Expert Panel: Improved glycaemic control with BIAsp 30 in insulin-naive type 2 diabetes patients inadequately controlled on oral antidiabetics: subgroup analysis from the IMPROVE study. Curr Med Res Opin 2009, 25:2643-2654
- Wood JR., Moreland EC., Volkening LK., Svoren BM., Butler DA., Laffel LM.: Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care 2006, 29:2355-2360
- Yang W., Gao Y., Liu G., Chen L., Fu Z., Zou D., Feng P., Zhao Z.: Biphasic insulin aspart 30 as insulin initiation or replacement therapy: the China cohort of the IMPROVE study. Curr Med Res Opin 2010, 26:101-107
- Yang W., Lv X., Li Q., Jia W., Tian H.: A prospective study to optimize insulin treatment by switching to insulin glargine in type 2 diabetic patients previously uncontrolled on pre-mix insulin: the optimization study. Curr Med Res Opin 2012, 28:533-541
- Yenigun M., Honka M.: Switching patients from insulin glargine-based basal-bolus regimens to a once daily insulin detemir-based basal-bolus regimen: results from a subgroup of the PREDICTIVE study. Int J Clin Pract 2009, 63:425-432
- Zick R., Petersen B., Richter M., Haug C.; SAFIR Study Group: Comparison of continuous blood glucose measurement with conventional documentation of hypoglycaemia in patients with Type 2 diabetes on multiple daily insulin injection therapy. Diabetes Technol Ther 2007, 9:483-492
- Zjačic-Rotkvić V., Cigrovski-Berković M., Grulović N., Baršić B.: Efficacy and safety of a basal-bolus regimen with insulin glargine in patients with type 2 diabetes after failing premix insulin therapy: A multicenter postmarketing study. Diabetol Croat 2012, 41:41-48
- Ceriello A., Cremasco F., Romoli E., Rossi A., Gentilella R.: Insulin lispro protamine suspension in the treatment of patients with type 1 and type 2 diabetes mellitus: a systematic review of published data. Expert Opin Pharmacother 2012, 13:255-281
- Chapman TM., Noble S., Goa KL.: Spotlight on insulin aspart in type 1 and 2 diabetes mellitus. Treat Endocrinol 2003, 2:71-76
- Davidson J., Vexiau P., Cucinotta D., Vaz J., Kawamori R.: Biphasic insulin aspart 30: literature review of adverse events associated with treatment. Clin Ther 2005, 27(Suppl B):S75-88
- Rys P., Pankiewicz O., Łach K., Kwaskowski A., Skrzekowska-Baran I., Malecki MT.: Efficacy and safety comparison of rapid-acting insulin aspart and regular human insulin in the treatment of type 1 and type 2 diabetes mellitus: a systematic review. Diabetes Metab 2011, 37:190-200
- 128.Valensi P: Biphasic insulin aspart 30/70 (BIAsp 30) in the treatment of type 1 and type 2 diabetes. Diabetes Metab Syndr Obes 2009, 2:61-71
- Velásquez-Mieyer PA., Neira CP.: Biphasic insulin aspart 30 for the treatment of type 1 diabetes mellitus. Expert Opin Pharmacother 2008, 9:2377-2382
- Boehm BO., Home PD., Behrend C., Kamp NM., Lindholm A.: Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in type 1 and type 2 diabetic patients. Diabet Med 2002, 19:393–399
- Roach P., Bai S., Charbonnel B., Consoli A., Taboga C., Tiengo A., Bolli G.; High Mix Study Group: Effects of multiple daily injection therapy with Humalog mixtures versus separately injected insulin lispro and NPH insulin in adults with type I diabetes mellitus. Clin Ther 2004, 26:502-510
- Chen J., Lauritzen T., Bojesen A., Christiansen JS.: Multiple mealtime administration of biphasic insulin aspart 30 versus traditional basal-bolus human insulin treatment in patients with type 1 diabetes. Diabetes Obes Metab 2006, 8:682–689
- Clements MR., Tits J., Kinsley BT., Råstam J., Friberg HH., Ligthelm RJ.: Improved glycaemic control of thrice-daily biphasic insulin aspart compared with twice-daily biphasic human insulin; a randomized, open-label trial in patients with type 1 or type 2 diabetes. Diabetes Obes Metab 2008, 10:229-237
- Mortensen HB., Aanstoot HJ., Annan F., Olsen B.: Biphasic insulin aspart 30 – treatment options in children and adolescents. Eur Endocr Dis 2006, 23-6
- Mortensen H., Kocova M., Teng LY., Keiding J., Bruckner I., Philotheou A.: Biphasic insulin aspart vs. human insulin in adolescents with type 1 diabetes on multiple daily insulin injections. Pediatr Diabetes 2006, 7:4-10
- Karagiannis T., Paschos P., Paletas K., Matthews DR., Tsapas A.: Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012, 344:e1369
- Bolen S., Feldman L., Vassy J., Wilson L., Yeh HC., Marinopoulos S., Wiley C., Selvin E., Wilson R., Bass EB., Brancati FL.: Systematic Review: Comparative Effectiveness and Safety of Oral Medications for Type 2 Diabetes Mellitus. Ann Intern Med 2007, 147:386-399
- Goto A., Arah OA., Goto M., Terauchi Y., Noda M.: Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013, 347:f4533