Comparator outcomes profile: need for an informed assessment of secondary outcomes for conducting post-launch observational studies in the HE&OR setting
-
Copyright
© 2015 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Sameer Gokhale |
Epidemiologist at EpiCube Innovations |
Introduction
Health economics and outcomes research (HE&OR) focuses to quantify the differential value that product brings to the patient [1]. Primary outcomes like efficacy and safety are of utmost importance and are well-researched for every molecule. Secondary outcomes are outcomes which are of secondary interest or that are measured at time points of secondary interest [2]. They provide additional value and help molecule distinguish itself from the competitor molecules in a competitive pharmaceutical industry. At times secondary outcomes can help increase confidence in the primary analysis by demonstrating consistence of effect across different outcomes [3]. There is a need for an informed assessment of secondary outcomes for key molecules vis-à-vis their competitor molecules in HE&OR setting. Policy-makers, clinicians, and patients increasingly seek information about comparator outcomes profile (COP) for decision making about treatment strategies. However analysis of secondary outcomes needs to be viewed with caution. Studies that are powered well to assess primary outcomes and not secondary outcomes should be avoided for this analysis [3]. It is possible that the design of the study is not appropriate for secondary outcome assessment and hence these studies can be excluded [3]. Unnecessary stratification of data to conduct sub-group analysis should be considered with extra caution [4]. Pooling summary statistics is useful only when researcher is dealing with relevant and homogenous studies with secondary outcome data of interest. It is important to peruse statistical methods section to decide which studies are eligible for COP analyses [5].
Objective
To identify prerequisites for constructing COP for a newer molecule in a specific disease indication and to showcase applications of COP in assessing value of the molecule vis-à-vis competitor molecules.
Steps in Developing Comparator Outcomes Profile
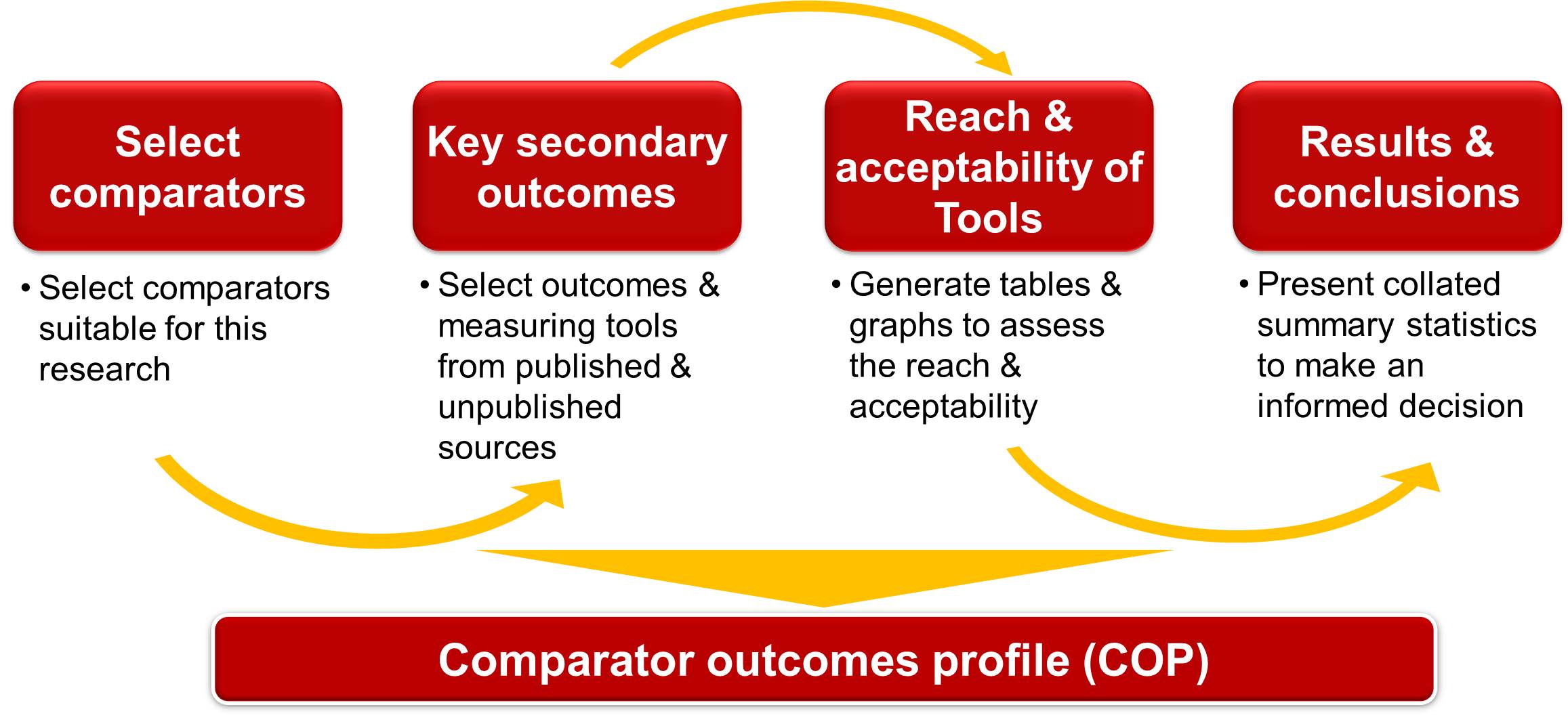
As shown in Figure 1, primary step is to select key comparators for a specific molecule in a particular disease indication. Gathering HE&OR specific secondary outcomes evidence from published as well as unpublished sources to select key outcomes is the next important step. Scientific articles published electronically in various scientific databases are perused to cull out relevant articles of interest. Studies reporting secondary outcomes from unpublished sources include those under active research in different research institutions (such as academic institutes/ public and private universities/ government establishments etc.) and are reported in publicly available clinical trial repositories such as ClinicalTrials.gov. Number of publications citing specified secondary outcomes across various countries will give insights for decision makers about secondary outcome’s global reach and acceptability among researchers/ patients/ stakeholders.
Steps in developing COP are detailed out in the following section:
Select Comparators
It is important that comparators chosen for this research should reflect clinically meaningful relationship with the specific molecule in question. Choosing the wrong comparator may introduce selection bias or it may introduce confounding by indication or severity. Thorough understanding of clinical practice, data sources, and statistical methods is necessary to realize the potential consequences of the comparator choices on confounding. Confounding by indication can be minimized by selecting comparators that has the same indications, similar contraindications, and similar adverse events if possible. To avoid confounding by severity, researcher need to keep in mind that certain drugs are used only to treat milder cases of the disease and are not used in severe cases. Finally it is crucial to select comparators keeping research hypothesis in mind so that researcher stay focused to identify solutions to questions. Recognizing the implications and potential biases associated with comparator selection is of utmost importance to confirm validity of COP analyses [6].
Select Key Secondary Outcomes
Effective and relevant secondary endpoints are a critical component of designing COP analyses. Clinical study must be powered sufficiently to detect a difference in primary as well as secondary endpoints. Subgroup analysis to interpret secondary outcomes needs to be conducted with caution as secondary outcomes do not have the same statistical authority as compared to primary outcomes [7]. Secondary endpoints are considered important to clinicians in helping identify the ideal treatment for their patients [8]. Secondary outcomes usually support primary outcomes in decision making. In other words, secondary endpoints are highly correlated with primary outcomes. Some of the examples of secondary endpoints are patient reported outcomes (PROs), quality of life (QoL), behavioral and cognitive scores, physician rated outcomes, and different survival endpoints (disease free, progression free, overall survival etc.).
Reach and Acceptability of Tools
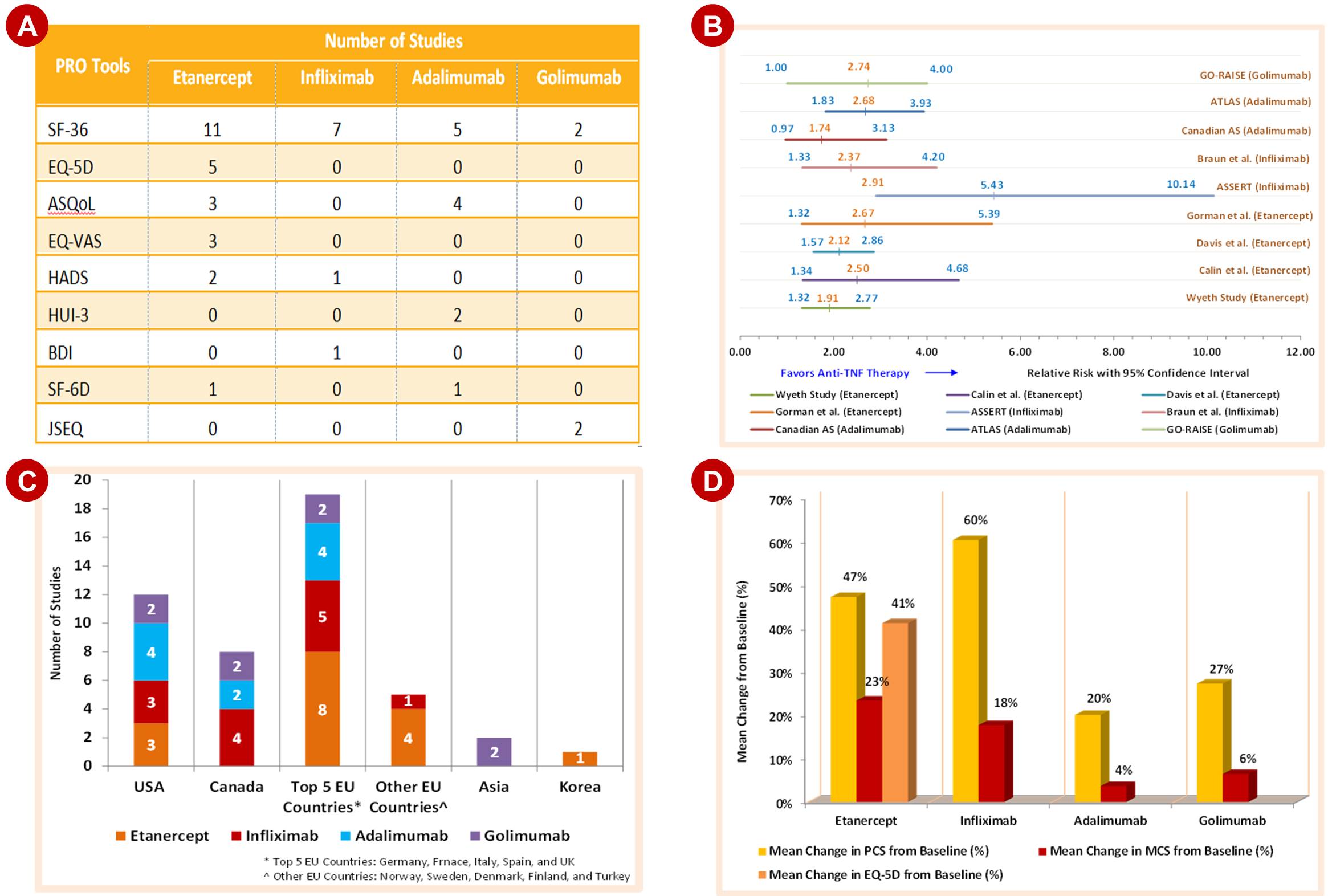
This step in development of COP is to validate whether the secondary outcomes selected are widely accepted by researchers across different countries of interest. As shown in Figure 2A, number of publications (published as well as unpublished) per comparator molecules are identified that are using select secondary outcomes such as PRO tools. Country wise number of relevant publications (as in Figure 2C) can also give confidence in selection of secondary outcomes for further comparison. Reach and acceptability of select tools are in direct correlation with the number of publications across comparator molecules. Once this validation is complete, next steps are to collate summary statistics which then can be compared to generate insights and recommendations.
Results and Conclusions
It is valuable to present collated summary statistics which will help decision maker to make an informed decision so that health providers and policy-makers can be offered optimal recommendations for patient care. As shown in Figure 2B & D, pooled relative risks from vital publications across comparators will give direction to the research hypothesis. Observations may find statistically significant change in patient outcomes or scores from relevant publications. These analyses will give direction to the healthcare stakeholders as to which comparator is outshining for a particular secondary outcome of interest.
Applications of Comparator Outcomes Profile
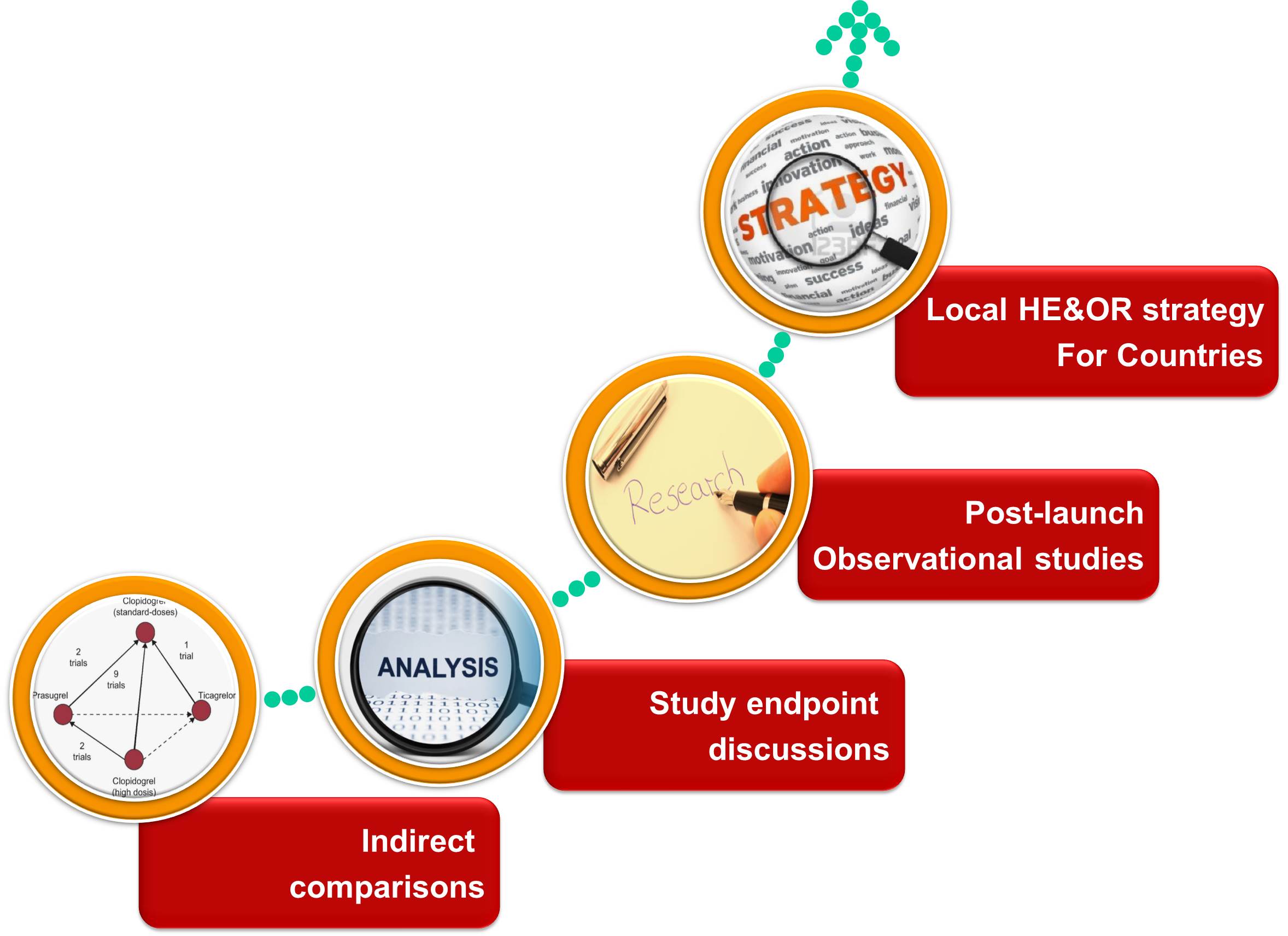
There are multiple applications of COP in assessing molecule’s value in the pharmaceutical industry. As shown in Figure 3, COP can provide guidance for indirect comparisons. Indirect comparisons are often used because of lack of, or insufficient, evidence from head-to-head comparative trials. Homogeneity of comparing studies is the basic assumption that should be taken care of while selection of studies so that indirect comparison can be used to provide insights [9]. Analysis of study end points and comparisons across relevant comparators can become a real-life evidence for strategic marketing for a pharmaceutical company. Company’s marketing and sales department can then use this information to prepare their country-wise brand plans for a particular molecule. COP can become a driving force for generating newer hypotheses which can help a brand grow exponentially. This is possible only if these hypotheses are tested successfully. Company can propose post-launch observational studies based on findings form COP which can be pursued further. COP can provide direction to the country organizations by providing strategic inputs tailored to suit respective country. Building country specific COP can be fruitful if factors such as disease epidemiology, disease severity, local clinical guidelines, and available relevant comparators are adjusted keeping in mind the specific country.
Conclusion
In summary, secondary outcomes are considered important to clinicians in helping identify the ideal treatment for their patients. Comparator outcomes profile document in health economics and outcomes research setting is designed to understand comparative clinical and health outcomes relevant to molecule’s value proposition against select comparators. Thus COP can be an important construct for defining and summarizing evidence on key secondary outcomes to assess molecule’s value.
- Whittington R. Introductions to Health Economics Concepts: A Beginner’s Guide. Greenflint Ltd. Flintshire, UK, 2008
- Sedgwick P. Primary and secondary outcome measures. BMJ 2010; 340: c1938
- Pocock Stuart J. Clinical Trials with Multiple Outcomes: A Statistical Perspective on their Design, Analysis, and Interpretation. Controlled Clin Trials 1997; 18: 530-545
- Freemantle N. Interpreting the results of secondary end points and subgroup analyses in clinical trials: should we lock the crazy aunt in the attic? BMJ 2001; 322(7292): 989–991
- Cohn JN. Introduction to Surrogate Markers. Circulation 2004; 109 (25 Suppl 1): IV20–1
- Velentgas P, Dreyer NA, Nourjah P, et al. Developing a Protocol for Observational Comparative Effectiveness Research: A User's Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Jan
- Wiebe S. The principles of evidence-based medicine. Cephalalgia. 2000;20 Suppl 2:10-3
- Rockhold F, Segreti T. Secondary Efficacy Endpoints in Encyclopedia of Statistical Sciences. John Wiley & Sons, Inc.; 2014
- Glenny AM, Altman DG, Song F et al. Indirect comparisons of competing interventions. Health Technol Assess 2005; 9: 1–134, iii–iv