RWE in Europe – An Analysis
-
Copyright
© 2018 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Background – This paper outlines findings from four roundtable discussions involving a number of stakeholders. They aimed to improve understanding of the use of real world evidence (RWE) across Europe. They focused on the development of a three-year roadmap for the increased incorporation of RWE, increasingly recognized as a valuable source of information for market access and reimbursement, in decision-making.
Methods – The meetings involved participants from 12 European countries. Participants had significant knowledge of specialist disease areas and commissioning of care and prior experience in the field of RWE. All four meetings involved plenary sessions with opportunity for discussion and feedback from participants. Specific topics of interest included the role of RWE in licensing, commissioning, clinical and patients and outcomes in chronic disease, oncology and rare diseases.
Results – We garnered significant insight into the current and future use of RWE across Europe, developing a three-year roadmap of initiatives for the enhanced use of RWE in decision-making. Four initiatives were seen to be the most important at this stage: actively engaging in early dialogue with payers on RWE needs; a consensus exercise on RWD/RWE in clinical decisions; developing a definition of Patient Reported and Patient Relevant Outcomes (PRO); and developing a model approach for the collection of PRO.
Conclusions – The roundtable discussions generated a wealth of information around the current and future value of RWE across Europe. Significant work is required in the areas of data generation, interpretation and use to make its inclusion in commissioning and licensing based decision-making more mainstream.
Introduction
Randomized controlled trials (RCT) have traditionally been seen as the ‘gold standard’ for drug approval data requirements but the use of real world evidence (RWE), derived from analysis of real world data (RWD) from sources such as electronic health records (EHR) and patient registries, is increasingly recognized as a valuable source of information for market access and reimbursement [1]. Potential issues with RCT, such as limited generalizability due to restrictive enrolment criteria, increasing complexity and the shrinking of potential populations for Phase 3 trials for orphan and rare disease treatments, may also be increasing interest in RWE.
For manufacturers, RWE may have the potential to solve problems inherent to getting a drug to market, despite issues around poor data quality and data security concerns. Using RWD could be a better means of proving the value of a new medicine to payers, resulting in quicker approval, more valuable discussions and the development of flexible reimbursement agreements, meaning that patients could access novel drugs at a sustainable price in a timely manner. Demonstration to stakeholders of the real life value of novel drug outcomes is key to improved patient access and manufacturer success.
There are a number of methodological challenges faced by those using RWE such as a lack of randomization, bias and issues around data quality [2]. One study has shown RCT data is still used in most health technology appraisals (HTA) – 90% of CADTH appraisals, 80% of PBAC appraisals, 64% of NICE appraisals and all IQWiG appraisals in 2015 used RCT-based evidence with only three positive appraisals based solely on non-RCT evidence [3].
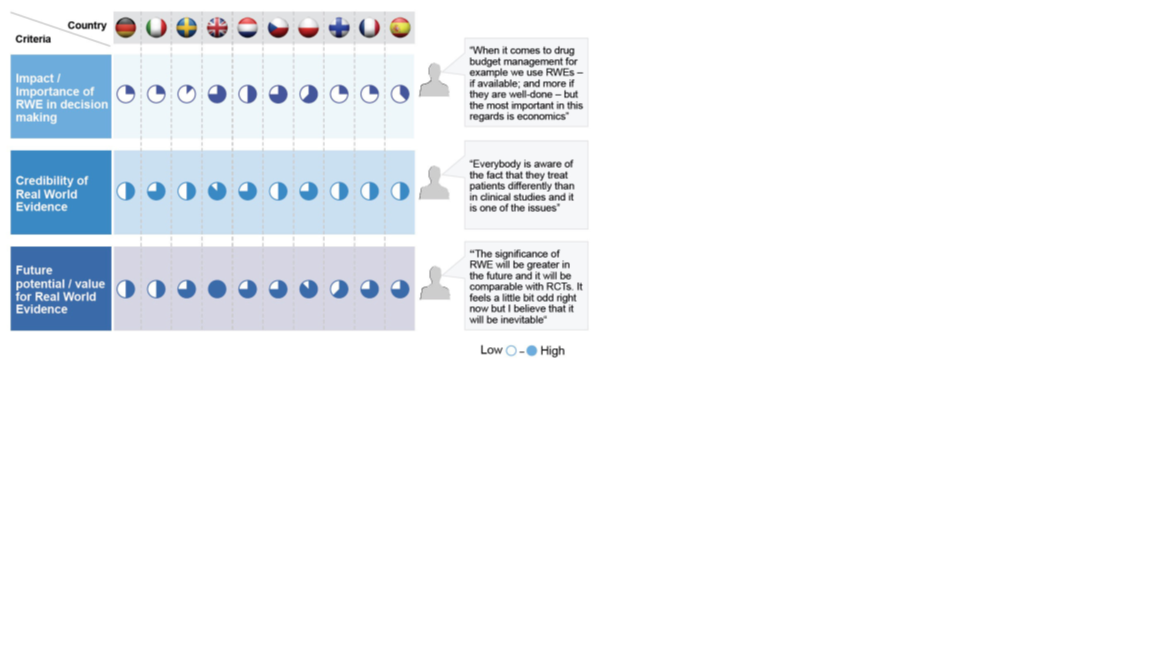
In 2015 increasing interest in RWE led to a “RWE voice of the customer project”, which gained insight into RWE needs and priorities across ten European markets. Figure one highlights the value and importance placed on RWE in each of these countries. The majority of countries see RWE as having medium-to-high future potential/value, but most thought that RWE had limited impact/importance in decision making at the time of survey completion (2015). The UK, France, Czech Republic and Poland saw slightly more value in RWE at the time.
Figure 1: Opinions of RWE use across ten European countries
Following this project additional analysis was required to get a more in depth understanding of the current use of RWE, as well as its future potential across Europe. This has taken the form of four round table style meetings throughout 2016 and 2017 with a group of expert stakeholders and key experts in pricing, reimbursement and RWE from a number of European countries. This paper summarizes the discussions held at these meetings. Whilst discussions were initiated and led by F. Hoffman-La Roche AG (referred to as “Roche” for the rest of this report), the results and action plans reported in this paper are applicable to all industry stakeholders aiming to enhance their use of RWE.
Materials and Methods
All four meetings involved a roundtable discussion with stakeholders with significant knowledge of specialist disease areas and commissioning of care and prior experience in the field of RWE. These experts represented Austria, Czech Republic, Denmark, France, Germany, Italy, Poland, Portugal, Spain, Sweden, Switzerland and the UK.
The first meeting, involving six participants, was held in London in June 2016. The aim of this meeting was to analyze the opinions of the key experts to get a more in-depth understanding of the current use of RWE in their respective countries as well as the future potential for RWE across Europe. Topics covered included: (a) Regulatory implications and the role of RWE; (b) RWE processes and implementation in decision making; (c) Meaningful outcomes from RWE; and (d) Priorities for focus and opportunities for industry cooperation and partnership.
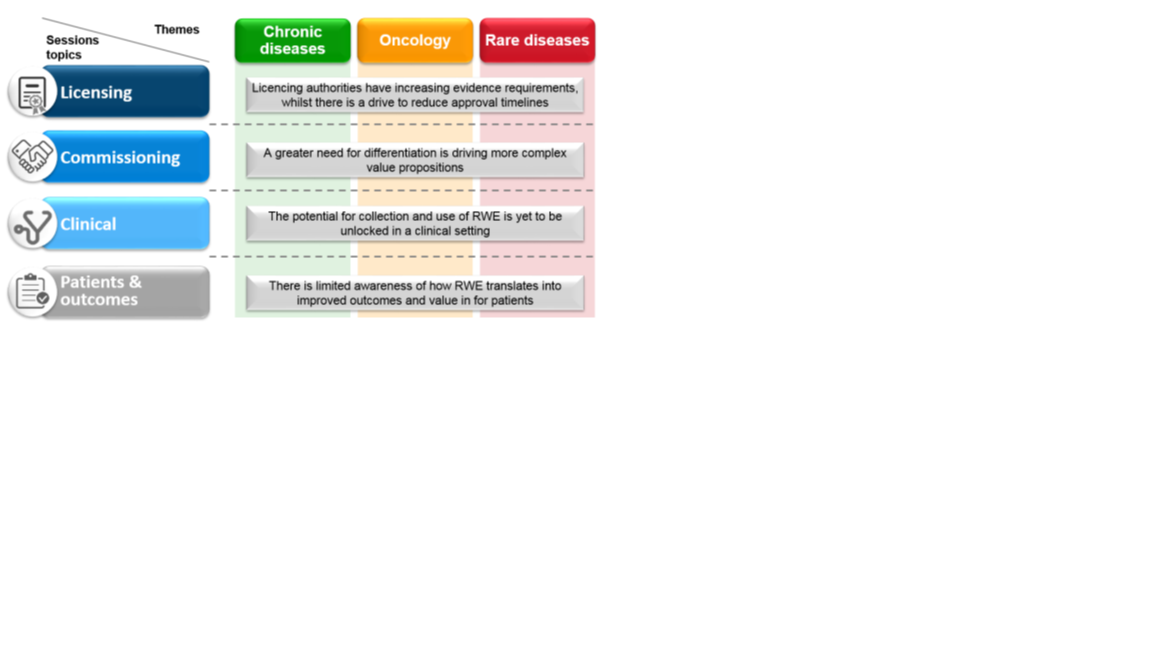
The second meeting, in December 2016, aimed to fill certain gaps highlighted following the first meeting. In order to gain further insight we included an additional six participants, some of whom had expertise in the area of patient organizations (PO). An hypothesis driven approach was used to explore the role of RWE in three treatment areas (chronic disease, oncology and rare diseases). Topics covered included: (a) Licensing; (b) Commissioning; (c) Clinical; and (d) Patients and Outcomes (Figure 2).
Figure 2: Meeting two session structure
Meeting three was held in Rome in June 2017. 15 participants, 12 of whom had been present at at least one of the previous meetings, built on discussion points from the two previous meetings to develop a 3-year roadmap of initiatives for the enhanced use of RWE in decision-making.
The fourth and final meeting was held in Zurich in October 2017 and included ten participants. This session centered on defining the future of the group by identifying ways to best utilize their capabilities to develop mutually beneficial and tangible outputs going forward.
Results
Output from the first meeting showed in general RWE is not used in regulatory (licensing) decision making but is used for accelerated regulatory review and conditional licensing, as well as re-review at year one, two or five with ongoing data collection.
It was not seen as a substitute for RCT, unless there was clear reason why the use of RCT was not feasible. There was some evidence that the paradigm may be changing. The increasingly chronic nature of some cancers is increasing the time taken to gather evidence on final outcomes, such as overall survival. RWE could play an important role in orphan conditions, where small patient groups may increase the length of time required for recruitment to traditional RCT. In some countries, like Poland, RWE can be considered equally important to classical experimental evidence. But problems lie in data availability with limited access to health quality data from EHR registries. In fact, sourcing data was seen as a general challenge by most of our expert panel. They confirmed the importance of encouraging a certain level of consistency across databases, with the ability to link data across countries and sectors to enhance data-usability. Again issues around patient confidentiality can play a role here. Registries should be designed and viewed as clinical trials with clarity in the questions asked of the registry, as opposed to focusing on the traditional ‘data fishing’ role of the registry.
RWE was recognized by all participants as a resource to support access decisions. However, this is not without its challenges, namely patient privacy and confidentiality requirements, as well as potential issues relating to recruitment to RCT if conditional approval of a drug is in place.
There is a clear role for RWE in reassessment/re-review, where it can address specific data requirements and end points. For example, it can confirm drug expectations including usage, efficiency, dose escalation and safety. It can be hard to reach an agreement on the type and quality level of data required for re-review processes as well as where responsibilities lie in terms of data collection. Early engagement would allow regulatory authorities to be explicit when outlining what data, both type and quality, is required for re-review.
As far as the role of industry was concerned, the first meeting highlighted their ability to ‘become the leader’, ‘develop the data’ and ‘create the community’. Industry could draw on internal expertise to use RWE to solve specific problems, and create mechanisms by which they can collaborate with decision makers in a more timely and effective manner. Industry could support formation and financing of registries and play a role in providing design expertise for registries, improving the quality and consistency of data collected, promoting the consistency and universality of use and address issues around confidentiality of patient data held within registries. Finally, they could promote collaboration between academic institutions, industry and health authorities to improve the credibility of RWE, and confidence in its use.
Meeting two focused on the role of RWE in licensing, commissioning, clinical decision-making and guidelines, and patients and outcomes across three condition areas. In licensing in the chronic disease arena, larger patient cohorts are available so RWE tends to be used secondarily to traditional RCT data. It is more likely that RWE will be used as a conditional requirement in licensing to support reimbursement. For rare disease, RWE may have more potential in licensing terms as there are situations where RCT is not feasible. However, the rare disease licensing system may not be ready for the contribution of RWE due to lack of available data and clarity on collection and funding responsibilities, there may be similar issues in the oncology arena.
In terms of commissioning, a greater need for differentiation is driving more complex value propositions. As chronic disease patients are living longer there is a paradigm shift in the way that commissioning (i.e. Financial decision-making) works and new commercial propositions such as pay for performance may be needed. As it stands, there is limited evidence that drug prices are affected by RWE.
For rare diseases there may be a specific role for patients within HTA decision-making to ensure the patient ‘voice’ is heard. Within this context there may be a significant role for PO, although their contributions vary across countries due to both ability and available resources. Industry should work towards integrating RWE into the HTA process, alongside RCT data, from the start, in the form of the value dossier. Data collection could be made mandatory by law, which would increase the volume available.
The patient is the underlying consistent factor in any clinical practice and their data should be used where possible. Using RWE has the potential to reduce treatment variability and prompt use of best practice (in conjunction with clinical guidelines) or manage clinical behavior. But, there is a requirement to collect RWE that addresses relevant issues, rather than just supporting or reinforcing RCT data. To get the best out of RWE there needs to be a concerted effort to collect RWD that addresses relevant real world issues, rather than just supporting or reinforcing RCT data, for example, by tracking patient co-morbidities, which may be challenging.
The importance of patient-shared decision-making is growing and it is vital to have useful, useable and relevant clinical data available for such decision-making. Similarly, patient-related data on side effect awareness and management could be vital for improving clinical decision-making. Such data can be collected via a combination of focus groups, surveys, patient forums and online evidence. Whilst patients are becoming more liberal in terms of data ownership and use there are still some issues around access to patient data that can limit evidence generation and the impact of RWE on clinical decision-making. This may be remedied by ensuring patients are educated on data confidentiality, as well as given the tools to allow them to better understand the HTA process, and their personal impact. This may ‘activate’ them to participate in RWE related research and data collection. PO have a role to play in training patients in RWE, and its benefits. Whilst some PO may be ready and willing to undertake training the variability in PO quality means that some will be much less willing and able to become engaged in such training opportunities.
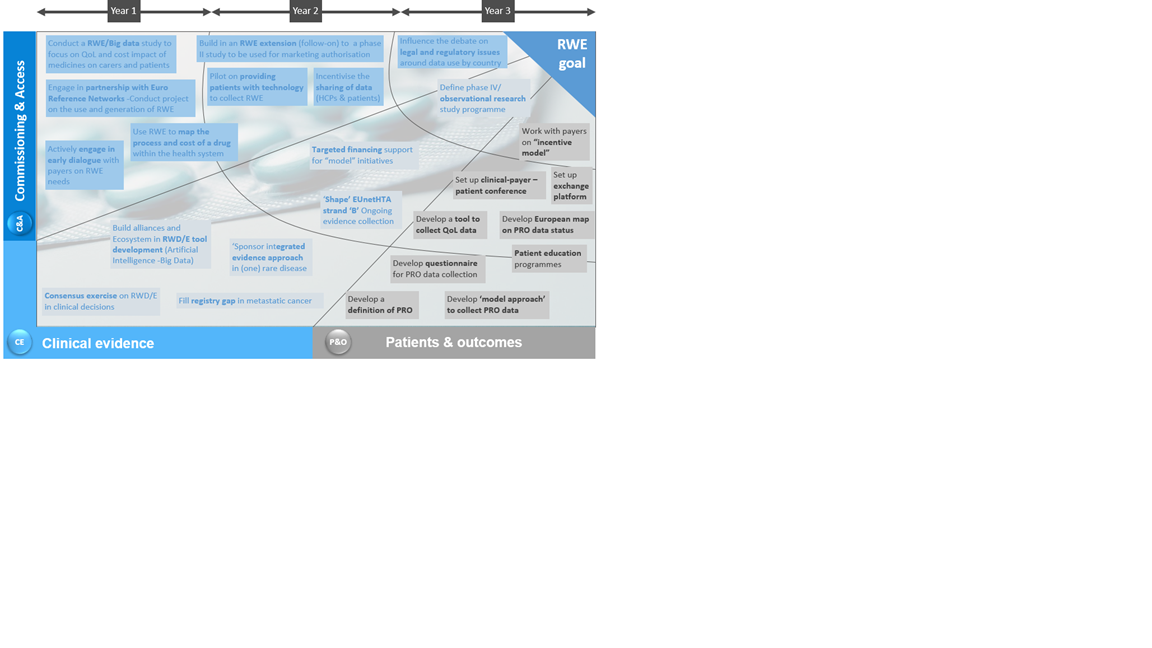
The third meeting focused on actionable results, building on discussion points from the first two meetings to develop a three-year roadmap of initiatives for the enhanced use of RWE in decision-making. Participants looked at an action-plan for the pharma industry across three areas of interest – commissioning and access, clinical evidence, and patients and outcomes.
A total of 24 initiatives of importance were identified by participants including issues such as developing a pilot looking at providing patients with technology to collect RWE and developing a tool to collect QoL data. These 24 initiatives were then prioritized in terms of importance for action over a three year period (Figure 3). Generally, those initiatives placed in year 1 were either thought to be the most urgent, or were seen as easier to complete than initiatives placed in year 3. Similarly, those initiatives placed in year 3 were seen to be less urgent, or required the completion of other initiatives before they could be tackled.
Figure 3: Three-year prioritization of 24 refined RWE initiatives identified by the expert panel
Stakeholders were asked to vote for what they saw as the most important areas for initial focus out of those initiatives in year 1. The top 4 initiatives were analysed in more depth. These included:
-
Actively engaging in early dialogue with payers on RWE needs – To develop better evidence packages to upskill payers. This will involve reviewing the use of RWE/RWD in early decision making nations (France, UK) via external and internal review, the development of processes (either separate or joint) with the EMA, or EUnetHTA, expansion of early dialogue to other markets where focus will be on comparator, on financing mechanisms and on sub-group data-options. Finally the targeted development of ‘Early Access’ evidence packages for specific products, as well as conducting education exercises for payers.
-
Consensus exercise on RWD/RWE in clinical decisions – To allow pharmaceutical manufacturers to define a process by which to develop clinically relevant RWE. This will involve an agreement on the definition of key data points used in RWE, generation of evidence in sub-populations not currently covered by existing trial populations, definition of the minimum standards for quality and methodology of data sets, ensuring robust data standards and methods, and developing or enhancing chronic disease management systems in terms of clinical pathways and practice guidelines.
-
Developing a definition of Patient Reported and Patient Relevant Outcomes – Lay foundations for empowering patients to engage more actively in the use of RWE. Enhance initial knowledge of existing definitions, approaches and best practices before development of research questions, priorities and research agendas
-
Develop a model approach for the collection of PRO data – Mapping a process for data collection, setting objectives for data collection and defining a model process accounting for data laws and any compliance issues and identifying any infrastructure/technology needs.
At the final meeting discussions moved towards implementation of these top 4 ‘year 1’ initiatives. First steps will involve mapping any ongoing RWE projects to increase understanding. It is vital to capture learnings from any initiatives in this way, as well as sharing experiences of best practice and advice developed as a result. Subsequently, developing a business case for pursuing the prioritized initiatives such as filling registry gaps in metastatic cancer - for example there may be an opportunity for the pharmaceutical industry to collect significant data related to breast cancer management. Furthermore, developing a definition of patient reported/relevant outcomes and developing a model approach to collect such data will be a focus. There is then a requirement to develop an implementation plan and identify the resources required, secure financial support, engage resources, key stakeholders and partners and finally implement individual projects.
In addition to the specific roadmap there is a place for pharma to develop standardized tools and data which can support various engagements in RWE. They can play a role in empowering and validating patient groups and organizations as well as clinicians – a key request from patients is for training, support and education in data collection. They can also assist with clearly defining outcomes for the disease area in focus, make data meaningful for all stakeholders and develop ‘smart data’ (digital information that is formatted so that it can be acted upon at the collection point before being transferred to an analytics platform for consolidation and analysis).
Discussion
This paper briefly outlines a number of discussions held between European-wide RWE experts over the course of 2016 and 2017. These discussions helped develop our understanding of the current role of RWE in both commissioning and licensing decisions. The benefits of RWE are becoming more prominent, and its use has already been put into practice in some markets such as the UK where it has been used to obtain evidence of drug use in a normal medical setting where an electronic patient data monitoring system, linking primary care, hospitals and pharmacies, was the key tool for data collection [4].
However, the landscape is currently fragmented and may require more focused leadership and collaboration across countries and institutions. There are still a number of questions to answer. For example, can the use of RWE address any gaps between licensing and commissioning?; how do we ensure that we are not asking RWE to do something that it is not designed for – what is the most appropriate role for this type of data?; and how do we incorporate patient data in the most effective way possible? Answering these questions may lead to the underpinning of a RWE strategy for the future.
Industry has a role in enhancing the use of RWE, and benefiting patients with earlier access to vital novel medicines. They can support better clinical practice via the use of RWE by backing the funding of patient registries to supply the all-important data, facilitating a dialogue between patients and decision makers, training stakeholders and linked PO in the importance of RWE and methods for its collection in order to build trust and confidence in RWE.
In terms of the future of this group of expert stakeholders, involved in the exploration of RWE across Europe since June 2016, there is the opportunity to improve and diversify their contribution by providing alternative avenues for research and support of local ideas and initiatives. They could play a role as a sounding board, provide valuable information and advice as well as feedback on the methodologies developed by pharma for the development of future RWE and its utilization.
Conclusions
In conclusion, the four stakeholder round-table meetings discussed in this paper have given us a wealth of information around the current and future value of RWE across Europe. Increased use of RWE is becoming more common and associated benefits more relevant, but there is no doubt that significant work is required in the areas of data generation, interpretation and use to make its inclusion in commissioning and licensing based decision-making more mainstream. This paper includes a number of proposed initiatives, with associated action points, that should be considered to further develop the use of RWE in decision-making.
Acknowledgements
The authors would like to thank the members of the advisory board discussion group whose thoughts and opinions feed into this paper.
Financial Support: This project was funded by a grant from F. Hoffmann-La Roche AG.
- Makady A, Stegenga H, Ciaglia A, Debray TPA, Lees M, Happich M, Ryll B, Abrams K, Thwaites R, Garner S, Jonsson P, Goettsch W on behalf of GetReal Work Packages 1 & 4 (2017). Practical Implications of Using Real-World Evidence (RWE) in Comparative Effectiveness Research: Learnings from IMI-GetReal. Journal of Comparative Effectiveness Research doi.org/10.2217/cer-2017-0044
- Pietri G. & Masoura P. (2014) Market Access and Reimbursement: The Increasing Role of Real-World Evidence. Value in Health 17, A450-A1.
- Griffiths E.A. & Vadlamudi N.K. (2016) Not ready for the real world? The role of non-RCT evidence in health technology assessment. Value in Health 19, A286.
- Neville S. (2017) GSK ‘real world’ study offers new model for drug trials. In: Financial Times, London