Availability of pneumococcal vaccination programmes in Europe. An overview of funding mechanisms
-
Copyright
© 2013 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Rafał Niżankowski |
Uniwersytet Jagielloński Collegium Medicum Wydział Lekarski; II Katedra Chorób Wewnętrznych |
|
Magdalena Koperny |
Dział Zdrowia Publicznego Wojewódzkiej Stacji Sanitarno-Epidemiologicznej w Krakowie |
|
Agnieszka Kargul |
Dział Zdrowia Publicznego Wojewódzkiej Stacji Sanitarno-Epidemiologicznej w Krakowie |
|
Michał Seweryn |
Zakład Ekonomiki Zdrowia i Zabezpieczenia Społecznego w Instytucie Zdrowia Publicznego. Wydział Nauk o Zdrowiu, Uniwersytet Jagielloński Collegium Medicum. |
Increasing antibiotic resistance is a global problem. Vaccinations is one of the best solution to reduce the burden of infectious diseases. Despite universal access to antibiotics, pneumococci still are one of the major causes of morbidity and mortality in both developing and developed countries. Criteria for making the decision on financing immunisation are similar in many countries and include identifying the target groups based on epidemiological risk, the clinical efficacy and safety of a vaccine, and the economic aspects of the implementation of vaccination. To reduce the risk of diseases caused by Diplococcus pneumoniae pneumococcal conjugate vaccine (PCV) is included in national immunisation programmes in most countries.
The paper describe international differences of organisations and funding mechanisms for pneumococcal vaccination. The analysis covered 34 countries in Europe. The study analyzed availability and type of vaccines used in vaccination programmes, children in the target age group to vaccination, vaccination financing, level of co-payment to vaccination for children and mechanism of purchasing vaccines.
In most European countries, PCV is included in national immunisation programmes. The differences regard the availability of two types of conjugate pneumococcal vaccines (10-valent and 13-valent). The method of recommending vaccines, mechanism of purchasing and funding in different countries is varies.
Introduction
The observed rapid technological progress in medicine is a continuous source of novel products, launched onto the market with newer and newer vaccines including combination vaccines. The availability of prophylactic vaccination programmes varies among European countries, being sometimes limited, while their selection depends on healthcare policy decisions in particular countries. Also the funding systems of vaccines are differentiated. Under certain universal vaccination programmes, patients may receive vaccines free of charge, while in other cases the costs of vaccines may be shared or fully incurred by patients. Following some literature reports, if vaccines are not funded from state budget or if some cost proportion is incurred by patients, as it happens in certain countries, it may directly compromise the availability of vaccines and vaccination programmes. Decision criteria, regarding the source of funding prophylactic vaccinations, are fairly similar in various countries and encompass the identification of target groups, based on epidemiological evidence of risks, the clinical efficacy and safety of available vaccines and the cost-effectiveness and pharmaco-economic aspects of assumed vaccination.
Despite universal access to antibiotics, pneumococci are still among major causes of morbidity and mortality, both in developing and developed countries. Due to the increasing resistance of pneumococcal strains to antibiotics, immunoprophylaxis becomes a more and more favoured option [1].
There are two types of antipneumococcal vaccines. The first one is the immunogenic pneumococcal polysaccharide vaccine (PPV23), approved to trading in 1980. It contains purified capsular polysaccharides of 23 pneumococcal serotypes and is administered in risk-group children above the second year of life, as well as in subjects above 65 years of life. In the year 2000, the other, conjugate vaccine, was launched onto the market and has been used ever since. Conjugate vaccines are new generation products, currently available in three forms: 7-valent pneumococcal conjugate vaccine (PCV7), 10-valent pneumococcal conjugate vaccine (PCV10) and 13-valent pneumococcal conjugate vaccine (PCV13,) consisting of antigens of specific pneumococcal, important from the epidemiological point of view. PCV7 vaccine has in most countries been replaced by newer generation products: PCV10 or PCV13 (available since 2009)  .
.
Following registered indications, PCV10 can be administered to infants and children from 6 weeks to 5 years of age, while PCV13 is indicated for infants, children, and adolescents of 6 weeks till 17 years of age and for adults over 50 years of age [2].
It has been shown that conjugate pneumococcal vaccines not only reduce the risk of diseases, caused by Diplococcus pneumoniae in vaccinated subjects, but they also offer a certain protection level to unvaccinated population, unless vaccination programmes are maintained at high enough strength and for satisfactory time period (herd immunity).
Epidemiology
In 2010, there were 21,565 cases of pneumococci-induced diseases, recorded in countries of the European Union and of the European Economic Area  , and the average morbidity rate was 5.22 cases per 100 thousand population for a total of 26 analysed countries. The highest percent of reported cases was noted in the Nordic countries (Denmark, Finland, Sweden, Norway) and Belgium. However, the presented data should be approached with caution, due the variability of monitoring systems and different scales of data completeness in particular countries. The highest prevalence of diseases of pneumococcal aetiology was recorded in the group of children at the age of 5 years (9.6/100,000) and in adults after 65 (14.4/100 000) [3].
, and the average morbidity rate was 5.22 cases per 100 thousand population for a total of 26 analysed countries. The highest percent of reported cases was noted in the Nordic countries (Denmark, Finland, Sweden, Norway) and Belgium. However, the presented data should be approached with caution, due the variability of monitoring systems and different scales of data completeness in particular countries. The highest prevalence of diseases of pneumococcal aetiology was recorded in the group of children at the age of 5 years (9.6/100,000) and in adults after 65 (14.4/100 000) [3].
Methodology
The assessment of solutions, accepted with regards to prophylactic vaccinations against pneumococci has been limited to the use of PCV10 and PCV13 vaccines in the population of children. Legal regulations, logistic organisation and the mechanisms of funding pneumococcal vaccination have been evaluated in 34  European countries. An analysis of prophylactic, anti-pneumococcal vaccination solutions was based on the following criteria:
European countries. An analysis of prophylactic, anti-pneumococcal vaccination solutions was based on the following criteria:
-
the availability and type of vaccines in vaccination programmes,
-
vaccination obligatoriness
-
vaccination funding sources,
-
vaccine purchase mechanisms,
-
location of patient access to vaccine
The national vaccination schedules, available at the ECDC (European Centre for Disease Prevention and Control) website, have been verified by interview, carried out in particular countries. Eurostat and ECDC databases were also searched to collect epidemiological data and healthcare costs in the analysed countries. Recommendations for vaccinations came from the website of the Centre for Disease Control and Prevention (CDC) or the ECDC and from the World Health Organization (WHO).
Recommendations
Following the recommendations of the American Advisory Committee on Immunization Practices (ACIP), regarding pneumococcal vaccination schedules for populations, aged from 0 to 18 years, a routine vaccination with PCV13 should be started in children at 2, 4, and 6 months of age (a three-dose protocol) with a booster dose to be administered between 12 and 15 months of age. Children between 14 and 59 months of age after a PCV7 vaccination series should receive one dose of PCV13, while in the catch up population  , one dose of PCV13 is recommended for all children between the 24th and the 59th month of life, which have not been covered by complete vaccination programme
, one dose of PCV13 is recommended for all children between the 24th and the 59th month of life, which have not been covered by complete vaccination programme  .
.
Regarding the prophylactics against infections with pneumococci, the WHO recommends PCV10 and PCV13 vaccines to be administered in children from the 6th week till the 5th year of life. Additionally, the 13-valent vaccine is recommended in adults at the age ≥ 50 years. The WHO recommends to include PCV vaccinations in national vaccination programmes, especially in these countries, in which the mortality rate of newborns is higher than 50/1000 live births  , however, each country, when designing a vaccination schedule, should take into account the actual morbidity rates and the age, at which the highest morbidity is recorded
, however, each country, when designing a vaccination schedule, should take into account the actual morbidity rates and the age, at which the highest morbidity is recorded  .
.
PCV in national vaccination programmes
At present, i.e., at the beginning of 2013, anti-pneumococcal conjugate vaccines of new generation are commonly available in all the 34 analysed countries of Europe. In 29 countries of the European market, both PCV10 and PCV13 are registered. Until the end of 2012, Synflorix (PCV10) had been available in 96 countries all over the world, including 22 countries of the European Union  .
.
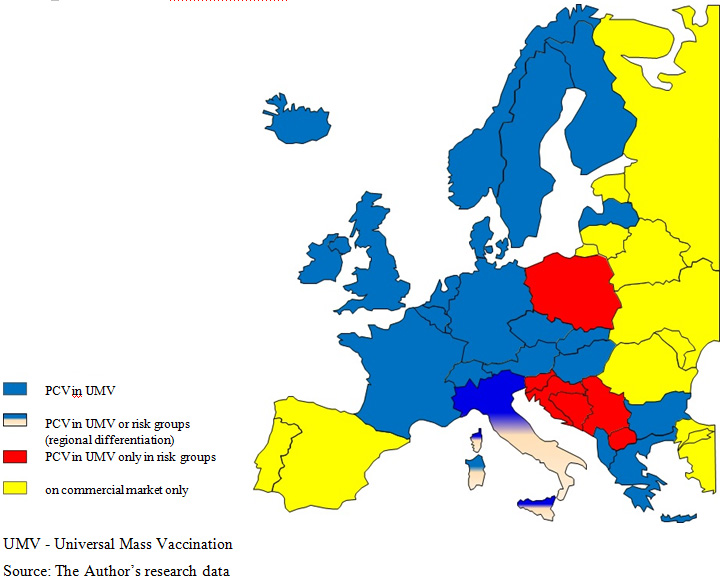
The proportion of vaccinations against pneumococci in national prophylactic programmes in Europe  is differentiated and biased by many factors, including epidemiological and demographic structures, the socio-economic level and the weight of prophylactics in health care system adopted in a given country [6]. As a rule, PCV vaccinations are part of national vaccination programmes, what can be observed in the prevailing majority of countries. If there are differences, they are mainly related to vaccine agents and vaccination target groups. In 6 countries: Spain, Portugal, Romania, Estonia, Lithuania and Malta, anti-pneumococcal vaccinations are available exclusively at the commercial market
is differentiated and biased by many factors, including epidemiological and demographic structures, the socio-economic level and the weight of prophylactics in health care system adopted in a given country [6]. As a rule, PCV vaccinations are part of national vaccination programmes, what can be observed in the prevailing majority of countries. If there are differences, they are mainly related to vaccine agents and vaccination target groups. In 6 countries: Spain, Portugal, Romania, Estonia, Lithuania and Malta, anti-pneumococcal vaccinations are available exclusively at the commercial market  . In Serbia, conjugate, 10- and 13-valent vaccines were included in the national vaccination programme in March 2013 and, until that time, they had been available at the commercial market, however, with a possibility to get full reimbursement from the Health Insurance Fund
. In Serbia, conjugate, 10- and 13-valent vaccines were included in the national vaccination programme in March 2013 and, until that time, they had been available at the commercial market, however, with a possibility to get full reimbursement from the Health Insurance Fund  .
.
It appears from the available data for 2013that in 19 countries, Synflorix  is applied via vaccination calendars or programmes, dedicated to risk groups. In 20 countries, Prevenar
is applied via vaccination calendars or programmes, dedicated to risk groups. In 20 countries, Prevenar  is the available vaccine, while in 10 other countries, (Croatia, Czech Republic, Greece, Germany, Macedonia, Slovenia, Slovakia, Sweden, Serbia
is the available vaccine, while in 10 other countries, (Croatia, Czech Republic, Greece, Germany, Macedonia, Slovenia, Slovakia, Sweden, Serbia  and Poland), both vaccines are used.
and Poland), both vaccines are used.
An analysis of public healthcare expenditure, expressed as a percentage of the gross domestic product (GDP), and of the implementation of PCV vaccination programmes by particular countries, taking into account vaccine types, did not show any relationship between the selection of more expensive (PCV13) or cheaper (PCV10) vaccine and the size of spending on healthcare. The Netherlands may be a good example, where healthcare expenditure in 2011 was the highest among the analysed countries, oscillating around 8.5% of the GDP, while the national vaccination programme included only PCV10, reimbursed from March 2010 and replacing PCV7 vaccine [8]. In turn, Slovakia, where the public spending for healthcare in 2010 was 5.9% of the GDP, both vaccines are in the national vaccination programme, with prevalence of the 10-valent vaccine [8]. In Poland, where the public spending on healthcare is one of the lowest (4.7% of the GDP), there is a free access of the risk group to the 10-valent vaccine and the 13-valent vaccine. In turn, both vaccines are recommended to and fully paid by other target groups, according to product characteristics. In Switzerland, where the public spending on healthcare is merely 2.1% of the GDP, vaccinations are funded under an immunisation programme for the entire cohort of children with a small, 10%, patient’s share in the costs. An analysis of public spending on healthcare, expressed in a proportion of GDP, and the general availability of immunising agents indicate that these are not the only factors which determine product funding by the public payer [8]. See Figure 1 for PCV availability in Europe.
Due to the economic reasons full funding of innovative vaccines is not always possible. One of the mechanisms to solve the problem of inability to provide immunoprophylaxis to entire cohort is to differentiate the availability of vaccines, offering free-of-charge or partially paid vaccines for particular, defined risk groups. In 23 countries, PCV has been included in national vaccination programmes for the entire cohort of patients, while in other, like Poland, Belgium, Croatia, Austria, Finland, the Netherlands, Germany, Iceland, Switzerland, Sweden, Bosnia and Herzegovina, and Serbia, vaccination is administered only to children in risk groups [9]  . In Portugal, vaccinations are free of charge only in hospitals and only with PCV13, while on the commercial market, both vaccines are available at full price for other target groups, either at pharmacies or Health Centres. On the other hand, both in Poland and Croatia, both vaccines are available and recommended in vaccination calendars, as well as on commercial market (in Croatia, Synflorix for children under two years of age, Prevenar13 over two years of age for the catch up population). In Italy, according to region, vaccinations are recommended for the entire population or only for patients at risk.
. In Portugal, vaccinations are free of charge only in hospitals and only with PCV13, while on the commercial market, both vaccines are available at full price for other target groups, either at pharmacies or Health Centres. On the other hand, both in Poland and Croatia, both vaccines are available and recommended in vaccination calendars, as well as on commercial market (in Croatia, Synflorix for children under two years of age, Prevenar13 over two years of age for the catch up population). In Italy, according to region, vaccinations are recommended for the entire population or only for patients at risk.
Vaccination schedules and target groups
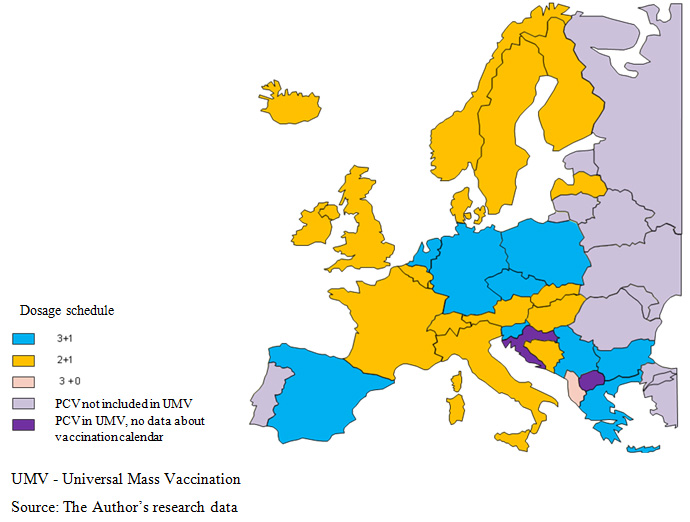
In countries, where pneumococcal vaccination is carried out under national programmes, the adopted vaccination schedules, which specify the number and timing of vaccine administrations, are varied. This situation may result from a diversity in epidemiological exposures, different in particular countries. In general, the following three vaccination schedules are applied 2+1, 3+1 and 3+0  . Albania is the only country, where the booster dose is not provided and children are vaccinated at the 2nd, the 4th and the 6th month of life [10]
. Albania is the only country, where the booster dose is not provided and children are vaccinated at the 2nd, the 4th and the 6th month of life [10]  .
.
In most European countries, the first vaccine in the 2+1 schedule is administered at the age of three months and the final dose at 5 months, while the booster dose is administered at 12 months of age (in Austria, Finland, Iceland, Italy, Sweden, Norway, Denmark and Slovakia the booster dose is recommended at 11 months). In the other countries on the 2+1 schedule, vaccinations are started at two months of life. The differences relate to time intervals between the priming doses (2 or 4 months) and the age of primary vaccination completion (11th – 15th month). In Romania, when the Ministry of health approves the new vaccination calendar, including anti-PCV agents, the vaccinations will be administered - similarly as in Belgium and the Great Britain – acc. to the 2+1 schedule  .
.
Primary vaccination in the 3+1 schedule is carried out in two variants in European countries: three doses, beginning in the 2nd month of life and continued with two-month (Greece, Spain) or one-month intervals (Bulgaria, the Netherlands, Germany), and only in the Czech Republic, vaccination starts at 3 months of life. Large discrepancies regard the time of booster dose administration, ranging from 11 to 18 months of life, depending on analysed country .
.
In Poland, basic vaccinations are also administered along the 3+1 schedule to high risk groups. The first dose is provided at 2 months of age with subsequent doses administered in one- or two-month intervals (3-4 and 5-6 months of life), while the booster dose is administered at 13-14 months of life. Various regimens of PCV administration do not delay the first dose of pneumococcal vaccine, which is administered between the 2nd and the 3rd month of life and, at the end of vaccination – up to the 6th month of life [9].
Vaccination funding
Compulsory vaccination programmes
In the prevailing majority of European countries, pneumococcal vaccination is not mandatory but recommended. Pneumococcal vaccination is compulsory for the entire population of children only in Greece, Bulgaria, and Slovakia. A specific situation is observed in Spain, where a conjugate vaccine is not included in the national vaccination program but in Galicia, PCV vaccination is obligatory for the entire cohort of children, while in other regions, there is a diversity regarding recommended target groups [11]  . In the regions, where vaccination is compulsory, it is completely free-of-charge for patients. In Slovakia, the more expensive vaccine (PCV 13) is available at 69% charge.
. In the regions, where vaccination is compulsory, it is completely free-of-charge for patients. In Slovakia, the more expensive vaccine (PCV 13) is available at 69% charge.
Recommended vaccination
In countries, where PCV immunisation is optional, there are several alternative forms of access to vaccines. Funding mechanisms and the rules of patient participation in the costs are controlled by the healthcare model, as adopted in a given country. Pneumococcal vaccination can be either free-of-charge for the entire population or for predefined risk groups only, but vaccination reimbursement programmes are also common, e.g. at pharmacies, vaccination centres or at GP’s / paediatrician’s offices.
In case of recommended vaccinations, high price is often a limitation for broad and effective PCV vaccination programmes. This is why countries exercise different ways to compensate restricted access, for example, by sharing costs between the patient and the public payer. Large differences are observed among particular countries in reimbursement levels for the patient. It should be emphasised that one of the key factors, which determine vaccination programme efficacy is its universal, free-of-charge and compulsory availability.
In the following six countries: Germany, Albania, The Netherlands, Slovakia, Serbia and Greece, anti-PCV vaccinations, being part of national vaccination calendars, are fully financed from the public funds. Serbia employs an unusual solution, where, until the time, when the recommended PCV10 and PCV13 vaccinations were included in the vaccination schedule, they had been available on the commercial market but with a possibility of full reimbursement for patients in risk groups. In Albania, PCV10 vaccines are 100% reimbursed for all the children, born after 2010, while for those, born before 2010, as well as for persons choosing PCV13 vaccine, there is no reimbursement. In Germany, the reimbursement level for the two vaccines is 100% for the child population, while Greece provides a full reimbursement of PCV13 vaccine for the entire cohort and of PCV10 vaccine for the subpopulation of children under 5 years only  .
.
Partial reimbursement is provided in 6 countries. The lowest co-payment proportion is in Switzerland, amounting to 10% for PCV13 vaccine, which is the only one used in that country. In Spain, vaccinations are free-of-charge in some regions, while in others, both conjugate vaccines are 40% payable. In France, the social security covers 65% of PCV13 cost, and PCV10 vaccine is not available on the market at all. A complex solution has been applied in Hungary, where the accepted schedule includes PCV13 vaccine with 70% reimbursement for children between 2 and 5 years of life and full payment for children over 5 and for adults. PCV10 vaccine is registered on the Hungarian market but is simply not available. See Table 1 for pooled data from countries with co-payment model  .
.
Table 1. Reimbursement levels in selected European countries for PCV 10 and PCV 13 vaccines
|
Country |
Vaccine type |
Reimbursement level |
Target group |
|
Switzerland |
PCV13 |
90% |
Entire cohort |
|
Slovakia |
PCV10 |
100% |
Entire cohort |
|
PCV13 |
69% |
No data |
|
|
Hungary |
PCV10 |
0% |
Entire cohort till the 2nd year of life |
|
PCV13 |
100% |
Entire cohort till the 2nd year of life |
|
|
70% |
Children till the 5th year of life |
||
|
0% |
Children after the 5th year of life and adults |
||
|
France |
PCV13 |
65% |
Entire cohort till the 2nd year of life and catch up children after the 2nd year of life |
|
Spain * |
PCV10 |
40% |
Risk group |
|
PCV13 |
40% |
Risk group |
|
|
Czech Republic |
PCV10 |
free-of-charge |
Risk group |
|
PCV13 |
lack of data |
Risk group |
|
|
Serbia** |
PCV10 |
100% |
Risk group |
|
PCV13 |
100% |
Risk group |
* regional differentiation; at certain autonomous regions vaccines are free-of-charge
** before March 2013, the vaccines had been available on the commercial market (beside the national vaccination programme)
Additional funding mechanisms
An additional mechanism to give patient a choice is to offer one vaccine free-of-charge, while other with partial or full payment. This solution was used in Slovakia and the Czech Republic: free immunoprophylaxis with PCV10 vaccine is recommended to the whole population, while the more expensive PCV13 vaccine is available with partial payment.
Alternatively, there are also local government prevention programmes that increase access of the local population to immunization, if vaccines are only available on the commercial market or are not covered by any compulsory programme. Such programmes are, among others, employed in Spain, Poland and Italy  . Moreover, local government programmes allow to cover with vaccination catch up populations, as it is, among others, in Italy .
. Moreover, local government programmes allow to cover with vaccination catch up populations, as it is, among others, in Italy .
Vaccines on commercial markets
The availability of vaccines, offered to the patient on the private market at full price, is found in some countries, together with vaccination in national programmes, e.g., in Bulgaria, Croatia, Poland, Lithuania, Romania, Bosnia and Herzegovina and, additionally, PCV7 vaccine is present on the market. In Hungary and Italy, PCV13 vaccine can be purchased at pharmacy (100% price) for children over 5 years old or for adults and in Hungary, also at vaccination centres. The situation is similar in Albania, with one difference that only PCV10 vaccine is available. In most cases, the option of product availability on commercial market correlates with the economic status of country, especially with the amount of spending on health care. Following an analysis of European countries, if spending on health care is below 6% of GDP, vaccines are available on the commercial market at full price [8].
Vaccine purchasing mechanism
In most European countries, the purchase and distribution of conjugate pneumococcal vaccines, used in national immunisation programmes or in local government programmes, are controlled by the law of public procurement, both in countries with high and low expenditures on health care  .
.
Vaccines may also be available via reimbursement lists. In Germany, Slovakia, Greece, and Serbia, 13- and 10-valent vaccines are on the list of reimbursed drugs and in Switzerland, the list includes PCV13 vaccine only. In France, Hungary, Italy and Spain, PCV13 vaccine is on the list of reimbursed medicines and can also be purchased via public procurement. The only possible way to purchase PCV10 vaccine in Spain is by invitation to tender. Sweden is the only one among the analysed countries, where both vaccines are on the list of reimbursed drugs and can be purchased by public procurement. A similar situation will take place in Serbia, including an implementation of the new vaccination programme, planned for August 2013. Differences in the vaccine purchasing mechanism in one country are associated with local vaccination policies adopted in particular regions of the country  .
.
In most countries, vaccines are acquired via public procurement at the central level. The availability of the 10-valent vaccine for the public health care system results from tender procedures in Latvia, Bulgaria, Finland, Albania, Iceland, The Netherlands and Cyprus, while the 13-valent is purchased by public procurement in Denmark, Portugal, Norway, the United Kingdom, Ireland and Hungary. In Macedonia, Slovenia, Croatia, and Poland, vaccines are purchased via central tender procedure, involving both types of vaccines. In Romania and Serbia, the updated vaccination programme is to include PCV vaccination and thus the purchase of those vaccines will be carried out via central tender procedures. In Croatia, an invitation to tender is announced every three years. Tenders for vaccines at regional level are announced in the following four countries only: Spain, Bosnia and Herzegovina (two tenders during a year), Sweden (in 10 regions) and in Italy (in 20 regions)  .
.
In Poland, vaccines are purchased in compliance with the Act of January 29, 2004 on the Public Procurement Law. A central invitation to tender is announced by the Department of Public Procurement – a unit, acting on behalf of the Ministry of Health [14].
See Table 2 below for detailed data of vaccine purchasing via public procurement in particular countries.
Table 2. Availability of vaccines via public procurement at central or regional level during the years 2009-2013
|
PCV 10 |
PCV13 |
|
|
2009 |
- |
Italy, Belgium |
|
2010 |
Bulgaria, Finland, Albania, Czech Republic, Cyprus, Sweden |
Denmark, Hungary, Czech Republic, Italy, Belgium, Ireland, Sweden |
|
2011 |
Bulgaria, Finland, Albania, Island, Czech Republic, Bosnia and Herzegovina, Cyprus Austria, Poland, Sweden, Croatia |
Norway, Ireland, The Netherlands, Hungary, Czech Republic, Italy, Belgium, Denmark, Poland, Sweden, Croatia |
|
2012 |
Austria, Bulgaria, Finland, Albania, Island, Czech Republic, Bosnia and Herzegovina, Cyprus, Poland, Sweden |
Macedonia, Norway, Ireland, Hungary Czech Republic, Italy, Belgium, Great Britain, The Netherlands, Denmark, Poland, Sweden |
|
2013 |
Latvia, Spain, Bulgaria, Finland, Albania, Island, Czech Republic, Sweden, Cyprus, Austria, Poland |
Macedonia*, Spain, Bulgaria, Norway, The Netherlands, Ireland, Czech Republic, Italy, Sweden, Bosnia and Herzegovina, Belgium, Great Britain, Denmark, Poland |
* Tender not yet announced
Analysis of vaccination coverage in the European population
Data on the level of vaccination coverage at the European level are limited and the lack of information from 22 analysed countries prevented the analysis from being complete and precluding key conclusions on the analysed topic. However, in data, published by the WHO  , a case of Germany is noteworthy as, despite widely available PCV13 and PCV10 vaccines for the entire population plus a 100% reimbursement of both vaccines and high spending on health care – the actual percentage of vaccinated subjects is relatively low. Moreover, the level of vaccination coverage varies much among the “Lender”, ranging from 23.3% (Hamburg) to 70% (Saxony-Anhalt) [13]
, a case of Germany is noteworthy as, despite widely available PCV13 and PCV10 vaccines for the entire population plus a 100% reimbursement of both vaccines and high spending on health care – the actual percentage of vaccinated subjects is relatively low. Moreover, the level of vaccination coverage varies much among the “Lender”, ranging from 23.3% (Hamburg) to 70% (Saxony-Anhalt) [13]  . A relatively low percentage of people are vaccinated in Latvia and Hungary, what may be due to low expenditures on health care vs. other, analysed countries (4.1 and 5.1% of GDP, respectively)
. A relatively low percentage of people are vaccinated in Latvia and Hungary, what may be due to low expenditures on health care vs. other, analysed countries (4.1 and 5.1% of GDP, respectively)  .
.
An analysis of the available data leads to a conclusion that the compulsory vaccination against pneumococcus in Slovakia results in the highest percentage of vaccinations (as much as 99%) which, in the future, will result in a high degree of resistance in the rest of the population. In other countries, where immunisation is not compulsory, vaccination coverage is lower than in Slovakia, but close to 90%. Table 3 below presents pneumococcal vaccination status during the years 2009–2011 [8].
Table 3. The percentage of subjects vaccinated during the years 2009 - 2011, 3 doses of PCV
|
Country |
2011 [%] |
2010 [%] |
2009 [%] |
|
Bulgaria |
94 |
69 |
bd |
|
Belgium |
no data |
84 |
84 |
|
Denmark |
90 |
88 |
86 |
|
France |
89 |
89 |
no data |
|
The Netherlands |
96 |
96 |
96 |
|
no data |
no data |
72 |
|
|
Hungary |
84 |
no data |
no data |
|
Ireland |
90 |
43 |
no data |
|
Latvia |
78 |
no data |
no data |
|
Norway |
92 |
91 |
91 |
|
Slovakia |
99 |
99 |
99 |
|
Sweden |
60 |
60 |
60 |
|
Great Britain and Northern Ireland |
90 |
89 |
no data |
Source: WHO Vaccine Preventable Diseases Monitoring System, http://apps.who.int/immunization_monitoring/en/globalsummary/countryprofileselect.cfm,
Rieck T. Impfquoten bei der Schuleingangsuntersuchung in Deutschland 2011. Epidemiologisches Bulletin 22. April 2013 / Nr. 16
Common availability of vaccination agents on domestic markets
Due to continuous changes, brought about by new technologies in the pharmaceutical sector, market shares of the analysed vaccines are very different in particular European countries. In 11 countries, the 13-valent vaccine has got a majority share, close to 100%. In Poland, since 2011, both vaccines have been available in national immunisation programmes and an analysis of the recent years shows an increased market share for PCV10 vaccine. Only in Finland, is the 10-valent vaccine recommended and free-of-charge for all children, having full market share and being funded since 2010. In Slovakia, Synflorix, introduced in 2011 to the national immunisation programme for children, has also got a predominant market share, while in Malta, pneumococcal vaccination is only available on the commercial market with recorded higher sales for the 10-valent vaccine. In Slovenia, Sweden, Serbia, and the Czech Republic, market shares of available vaccines (free of charge or with partial charge) are very close. The situation is similar in Estonia and Romania, although vaccines in these countries are only available at full price on the commercial market  .
.
Conclusions
An analysis of pneumococcal vaccination systems in Europe shows significant differences among countries and in many respects, mainly in recommended vaccination schedules and target groups. Despite varying vaccination schemes, the age, when the first vaccine dose and the booster dose are administered is similar in most European countries. The differences are related to the availability of two conjugate pneumococcal vaccines (10-valent and 13-valent), included in vaccination schedules. Vaccine recommendation apparatus is different in particular countries. Vaccination is compulsory in a few countries and, in some other countries, there are regional differences in vaccination scope and target group definition. Advisory committees, which define national vaccination schedules, do not always recommend the type of vaccine to be administered to patients, in some countries, for example, the decision belongs to a general practitioner/paediatrician or the patient himself/herself. The choice of vaccine is often associated with patient's participation in the cost of more expensive vaccines.
In countries with decentralised administrative structure, such as Germany, Spain, Italy, or Sweden, the scope of recommended vaccinations may differ in autonomous regions. The registration and approval of vaccines on the market are controlled by the central government, while the purchase of vaccines is usually arranged by public procurement at regional levels. Despite that, the vast majority of European countries demonstrate a centralised policy of preventive immunization, based on the public payer system. In a few countries, PCV vaccinations are on the list of reimbursed drugs, but the central procurement by the Ministry of Health is definitely a more widespread mechanism The inclusion of PCV vaccination in the national schedule – or its lack - does not depend directly on the socio-economic status of a country and may result from habitual and system tradition. Despite that, in 90% of cases, PCV13 vaccine is funded by the public payer. The cost-effectiveness of immunisation is one of the criteria for decisions, although not the only one. The type of available and funded vaccine is not fully dependent on the level of expenditure on health care in the analysed European countries, as supported by analyses of health expenditures, expressed in percentage of GDP. The patient co-payment model is – in case of vaccines – more common in countries with lower spending on health care, except France (8.3% of GDP), where there is surcharge for PCV13 vaccine. The evaluation of the efficacy of the studied vaccines is not presented in this study, as the vaccines have been used only recently on different national markets, while benefits from immunoprophylaxis may be assessed after several years only [5]  .
.
In conclusion, a trend is observed in European countries towards updating and expanding national immunisation programmes by innovative immunising agents and via public health care systems, although the funding mechanisms for PCV vaccination are diversified, being in most countries controlled by decisions of the central government.
Disclosures
The study was sponsored by GlaxoSmithKline Pharmaceuticals SA
- Atkinson W., Wolfe Ch., Hamborsky J. Epidemiology and Prevention of Vaccine-Preventable Diseases The Pink Book. Centers for Disease Control and Prevention eds. 12th ed., second printing. Washington DC: Public Health Foundation, 2012; 233-246
- Summary of Product Characteristics Prevenar 13. Available from: http://www.ema.europa.eu/docs/pl_PL/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf [Accessed: 28.03.2013]
- Fraser G., Hruba F., Quinton Ch., Albu C. Annual Epidemiological Report 2012. Reporting on 2010 surveillance data and 2011 epidemic intelligence data. Stockholm; ECDC 2013
- Akinsanya-Beysolow I., Jenkins R., Meissner C. Advisory Committee on Immunization Practices (ACIP) Recommended Immunization Schedule for Persons Aged 0 Through 18 Years - United States, 2013
- Pneumococcal vaccines WHO position paper-2012. Wkly Epidemiol Rec 2012; 87(14): 129–144
- Cylwik A., Grzesiowski P., Parda R., Proposed amendments to the national immunisation system aimed to develop access to vaccination in Poland. Infection Prevention Institute. Warsaw, November 2012.
- Pneumococcal vaccination (PCV) overview in European countries. EUVAC. net A surveillance community Network for vaccine preventable infectious diseases. Available from: http://www.euvac.net/graphics/euvac/vaccination/pcv.html; [Accessed: 06.04.2013]
- Freysson L., Wahring L. General government expenditure in 2011 – Focus on the functions ‘social protection’ and ‘health’. Economy and Finance. Eurostat Statistics in focus 9/2013
- Statement of the Chief Sanitary Inspector of 29 October 2012 on Preventive Immunisation Programme in 2013, Journal of Laws 2012 item. 78
- Nelaj E, Bino S, Preza I, Albanian vaccination coverage 2009-2010, Institute of Public Health. Available from: http://ecdc.europa.eu/en/eurovaccine/Documents/Eurovaccine_2011_Nelaj.pdf ; [Accessed: 20.04.2013]
- Moreno-Perez D., Alvarez Garcia .J., Aristegui Fernández J. Immunization schedule of the Spanish Association of Pediatrics: 2012 recommendations. An Pediatr (Barc). 2012; 76(1): 43
- WHO Vaccine Preventable Diseases Monitoring System. Available from: http://apps.who.int/immunization_monitoring/globalsummary/; [Accessed: 16.04.2013]
- Rieck T. Impfquoten bei der Schuleingangsuntersuchung in Deutschland 2011. Epidemiologisches Bulletin 22 April; 16/2013
- Act of 29 January 2004 Public Procurement Law Dz. U. of 2004 No. 19 item. 177 as amended.