Economic evaluation of acute lymphoblastic leukaemia treatment with clofarabine (Evoltra®) combined with chemotherapy for children and adolescents in Poland
-
Copyright
© 2012 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Background: To evaluate the cost-effectiveness of using clofarabine (Evoltra®) in combination chemotherapy (cyclophosphamide + etoposide) vs. nelarabine and IDA-FLAG protocol (idarubicin, fludarabine, cytarabine, GCSF - Granulocyte Colony Stimulating Factor, cytarabine) for the treatment of acute lymphoblastic leukaemia among children and adolescents who have relapsed or are refractory, after receiving at least two prior standard cyclesregimens and in casewhere there areis no other options enablingtreatment option anticipated to predictresult in a long-termdurable response (as a third-line therapy, used among people qualified for patients qualifying for hematopoietic stem cell transplantation), in the Polish setting.
Methods: The economic analysis was conducted on the basis of results of a systematic review. The cost analysis was carried out from the public payer (the National Health Fund) perspective in Poland, assuming that Evoltra® is financed from public funds within the chemotherapy catalogue. Direct medical costs were taken into account. Medical resources usage was determined on the basis of the results of a survey conducted in Polish centers specialized in haematology and children’s oncology. The time horizon corresponds to the patients’ life expectancy. Costs and health effects were discounted at 5% and 3.5% rate, respectively. The cost threshold for an additional quality-adjusted life year (QALY) is in line with the requirements of the Agency for Health Technology Assessment and amounts to PLN 99,543.
Results: Gaining an additional life year due to the use of clofarabine instead of nelarabine and IDA-FLAG protocol is associated with the cost from the public payer perspective PLN 27,529 and 26,046 respectively within the lifetime horizon. Cost of additional quality-adjusted life year (QALY) has been estimated at PLN 32,600 and 30,336 respectively. The results of probability sensitivity analysis confirmed stability of conclusions from the basic analysis.
Conclusions: Clofarabine (Evoltra®) used in combination chemotherapy for recurrent or refractory acute lymphoblastic leukaemia in children and adolescents is highly cost-effective therapeutic option in Poland compared with nelarabine and IDA-FLAG regimen.
Introduction
Acute Lymphoblastic Leukaemia (ALL) is a cancer deriving from progenitor cells of the hematopoietic and lymphatic system [1]. The disease develops as a result of cancer cells proliferation in bone marrow and displacement of normal haematopoiesis or as a result of cancer cells accumulation in other organs outside bone marrow. In Poland, leukaemia account for 30% of all cancers among children, whereas acute lymphoblastic leukaemia account for 75%-80% of all forms of leukaemia among children aged less than 18 and approximately 20% of all types of leukaemia among adults [2,3]. Peak incidence occurs between age of 2 and 5 years.
Direct causes of acute lymphoblastic leukaemia are not fully known yet. Potential factors contributing to the development of the disease are: exposure to pathogenic agents, the influence of the environment and genetic predisposition [1,4,5]. In most patients non specific symptoms are reported (weakness, infection with fever and inflammatory lesions or even abscesses in the nasopharynx, loss of appetite) 2-6 weeks before the confirmed diagnosis [2].
Treatment leads to a complete remission in more than 95% of patients, however, approximately 20-30% of patients suffer from a relapse which remains the most frequent reason of a treatment failure and is associated with poor prognosis. Relapse of acute lymphoblastic leukaemia results from clones of the remaining leukemic blasts resistant to the first-line treatment. The blasts biological characteristics, including immunophenotype, might have a big impact upon selecting the right treatment strategy [4]. Thus relapse of acute lymphoblastic leukaemia poses a serious clinical problem. In Poland, the first relapse of ALL is diagnosed in 30–40 children every year.
In an acute lymphoblastic leukaemia therapy following the failure of all prior treatments for children and adolescents, clofarabine (Evoltra ®), nelarabine and IDA-FLAG chemotherapy regimen (idarubicin, fludarabine, cytarabine and a granulocyte colony-stimulating factor (G-CSF)) are currently used in Poland.
According to the epidemiology data presented in reports of Oncology Center in Warsaw the incidence rate for lymphoid leukaemia (including lymphoblastic leukaemia) was 3.2/100,000 among boys aged up to 19 and 2.8/100,000 among girls aged up to 19 [6] in 2009 [6]. Based on the epidemiology data in Poland and around the word the disease is categorized as rare. Rare diseases are often life-threatening or chronically debilitating, constituting a serious health problem for the society and are considered a priority in EU health and scientific research programs [7,8]. Usually there is no effective treatment, but screening for early diagnosis, followed by suitable care, can improve quality of life and life expectancy.
Medicinal products used in rare diseases are commonly referred to as orphan drugs.
On February 2, 2002, Evoltra ® was labeled as an orphan medical product used for treatment of acute lymphoblastic leukaemia in paediatric patients who have relapsed or are refractory after receiving at least two prior regimens and where there is no other treatment option anticipated to result in a durable response [9].
The aim of this analysis is to evaluate the cost effectiveness of using clofarabine (Evoltra ®) in combination chemotherapy (cyclophosphamide + etoposide) vs. nelarabine and IDA-FLAG protocol (idarubicin, fludarabine, cytarabine, G-CSF) for recurrent and refractory acute lymphoblastic leukaemia among children and adolescents, after receiving at least two prior standard lines and in case there are no other options enabling to predict a long-term response (as a third-line therapy, used among patients qualified for hematopoietic stem cell transplantation), given the Polish market conditions.
The cost-effectiveness evaluation of clofarabine includes most common therapeutic scheme in Poland, i.e. clofarabine combination treatment according to the Locatelli’s scheme which encompasses clofarabine (a dose of 40 mg/m2), cyclophosphamide (400 mg/m2) and etoposide (150 mg/m2), used for 5 consecutive days as an intravenous infusion [10]. Locatelli’s scheme is recommended by the Polish (Polish Union of Oncology 2011) and international (National Cancer Institute 2012) guidelines for clinical practices for patients when there is no response to acute lymphoblastic leukaemia relapse treatment among children and adolescents [11,12].
Materials
The economic assessment was based upon a clinical effectiveness analysis using a systematic review approach. As part of the clinical effectiveness analysis, after reviewing medical databases, studies were qualified for analyses based on their subject matter and reliability. Then clinical effectiveness and safety results were combined with each other for individual methods of handling patients from the group analysed. The evaluation of clinical data credibility was carried out pursuant to the principles of Evidence-Based Medicine (EBM) [13].
Clofarabine’s clinical effectiveness assessment was carried out in comparison to the reimbursed technologies:
- chemotherapy according to IDA-FLAG protocol - for T-cell and B-cell lymphoblastic leukaemia;
as well as compared to
- nelarabine which may be considered a comparator for clofarabine only in case of T-cell acute lymphoblastic leukaemia due to its registered indication for treatment of patients with T-cell acute lymphoblastic leukaemia (T-ALL) and a T-cell lymphoblastic lymphoma.
Methods
RESEARCH TECHNIQUES
Based on the results of the systematic review, a cost effectiveness analysis and a cost utility analysis were performed for using clofarabine in combination chemotherapy (cyclophosphamide + etoposide) vs. nelarabine and IDA-FLAG.
The results of a systematic review indicated lack of studies directly comparing clofarabine with any alternative therapy in the analysed indication and lack of clinical studies concerning the use of clofarabine compared with other drugs which might potentially be used as a common comparator for an indirect comparison (clinical studies for the clofarabine found while examining medical databases were without clinical control group).
Given the above, and due to the fact that clofarabine is an orphan drug it was not possible to conduct a standard direct or indirect comparison, a clinical analysis was carried out using an indirect comparison without adjustment towards a common comparator (the so-called “naive” indirect comparison). Pursuant to the Minister of Health’s Ordinance of April 2, 2012, the analysis includes a list of data from separate clinical studies for clofarabine, nelarabine and chemotherapy according to IDA-FLAG protocol, compared against a natural course of the disease (understood as lack of causal treatment, i.e. applying placebo or the best available palliative care) [14].
The economic analysis comprises the following assessment:
- A list of health results and the costs of using comparable methods as part of the so-called “naive” indirect comparison
- A calculation of cost-effectiveness and cost-utility ratios for the technology and optional technologies.
Within the cost effectiveness and cost utility analysis the costs per life year gained (LYG) and quality adjusted life year (QALY) gained were calculated in case of replacing nelarabine or IDA-FLAG with clofarabine (Evoltra ®), in children and adolescents with recurrent or refractory acute lymphoblastic leukaemia after using at least two standard treatment cycles (as part of the third-line therapy), over a lifetime horizon, from the public payer perspective in Poland.
THE DESCRIPTION AND ASSUMPTIONS OF THE MODEL
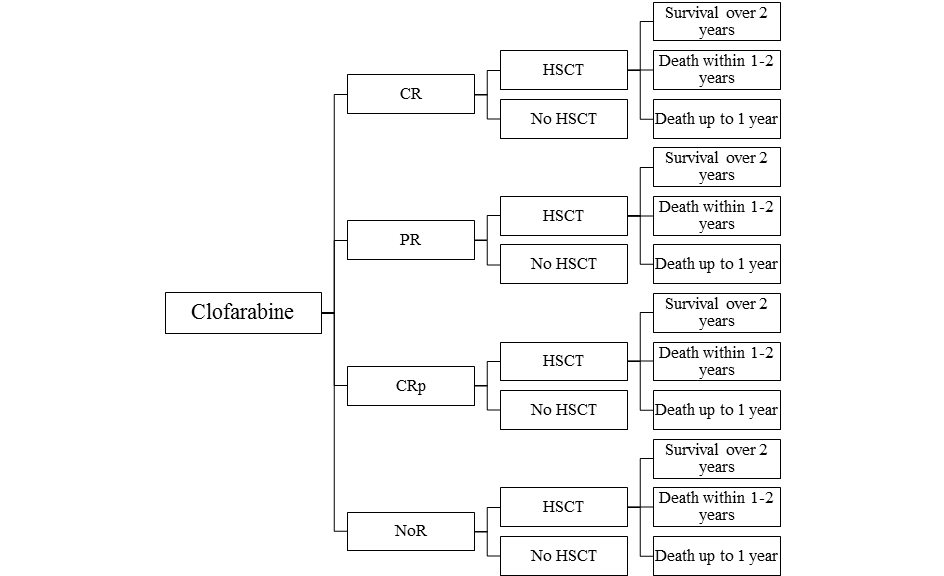
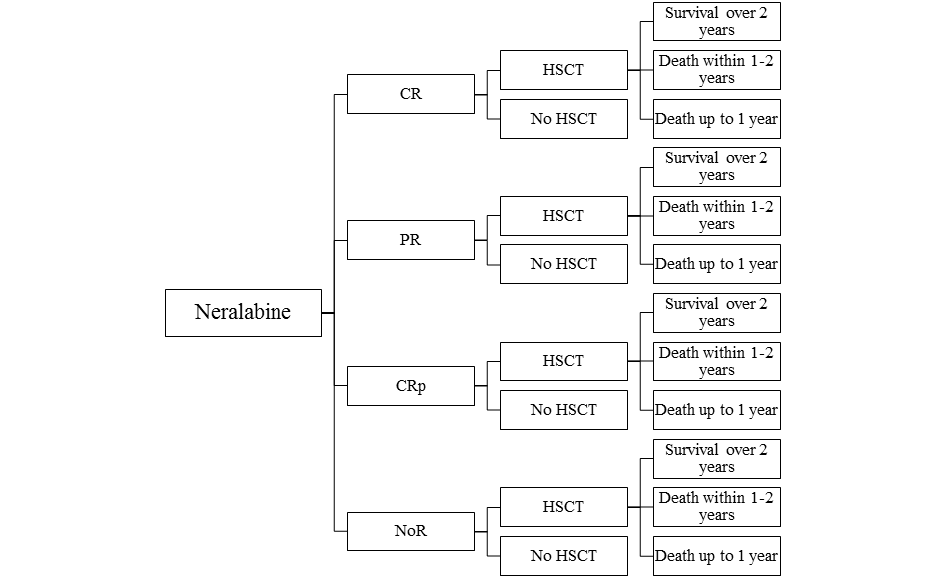
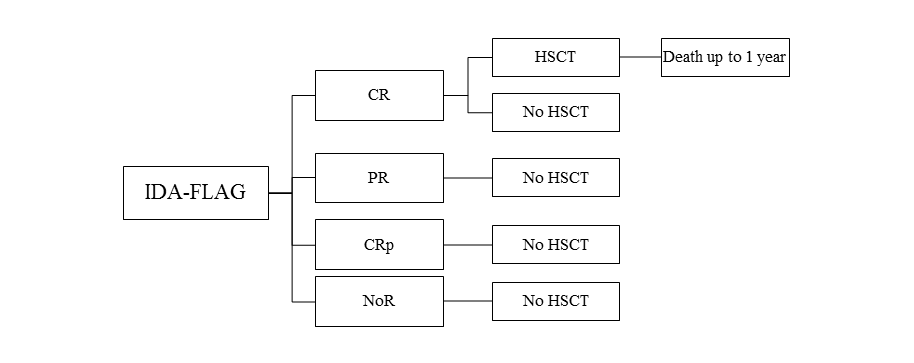
The model assumes that a patient treated for recurrent or refractory acute lymphoblastic leukaemia among children and adolescents, after using at least two previous standard treatments and in case there are no other options enabling the prediction of a long-term response within the third line of therapy, receives clofarabine (Evoltra ®) in combination with chemotherapy, nelarabine or IDA-FLAG. Using clofarabine, nelarabine or IDA-FLAG within the third course of therapy of acute lymphoblastic leukaemia may result in a complete remission (CR), a partial remission (PR), a complete remission without platelet recovery (CRp) or no objectively verified response (NoR). After the use of clofarabine, nelarabine or IDA-FLAG regimen, the patient may have received the hematopoietic stem-cell transplantation (HSCT). With regard to patients who received clofarabine, nelarabine or chemotherapy according to IDA-FLAG protocol within the third course of therapy of acute lymphoblastic leukaemia and then received HSCT and lived for over 2 years after the transplantation, the life expectancy after HSCT was based upon the expectancy for a person from the general population of Poland, with the same age as the patient. Patients who received clofarabine, nelarabine or IDA-FLAG within the third course of therapy of acute lymphoblastic leukaemia and then were subject to HSCT were considered to live over 2 years, 1-2- years, or up to 1 year. Survival of the patients either those who were subjected to HSCT or were not subjected to HSCT was based on the studies: clofarabine, nelarabine and IDA-FLAG [10,15,16,17,18,19,20]. Based on study only one patient with ALL that received IDA-FLAG had CR and HSCT and survived 2 months (the decision tree Figure 3. for IDA-FLAG treatment reflects the patients disease course from the study [20]).
A simulation was conducted using a decision-tree with time horizon determined at the level of life expectancy of a patient from the analysed population. The scheme of the decision model trees considered in the analysis is shown in the figures (Figure 1, Figure 2, Figure 3).
CLINICAL DATA
The probabilities and data sources for the occurrence of a response to treatment, the probability of receiving HSCT and the general survival after HSCT following the application of the schemes are shown in Table 1.
Table 1. Health response for clofarabine combination therapy, nelarabine, IDA-FLAG
| Parameter | Mean | Lower Confidence Interval [LCI] | Upper Confidence Interval [UCI] | Source | |
| % of patients with treatment response | |||||
| Clofarabine combination therapy | CR | 52.00% | 32.82% | 70.88% | [10] |
| PR | 8.00% | 1.03% | 21.12% | ||
| CRp | 4.00% | 0.11% | 14.25% | ||
| Nelarabine | CR | 12.82% | 4.41% | 24.80% | [15, 19] |
| PR | 2.56% | 0.07% | 9.25% | ||
| CRp | 10.26% | 2.94% | 21.38% | ||
| IDA-FLAG | CR | 22.22% | 3.19% | 52.65% | [20] |
| PR | 11.11% | 0.32% | 36.94% | ||
| CRp | 0.00% | - | - | ||
| % of patients who underwent HSCT | |||||
| Clofarabine combination therapy | CR | 53.85% | 27.67% | 78.91% | [10] |
| PR | 0.00% | - | - | ||
| CRp | 0.00% | - | - | ||
| NoR | 0.00% | - | - | ||
| Nelarabine | CR | 60.00% | 19.41% | 93.24% | [15, 16, 17, 18, 19] |
| PR | 100.00% | - | - | ||
| CRp | 25.00% | 0.84% | 70.76% | ||
| NoR | 10.34% | 2.27% | 23.50% | ||
| IDA-FLAG | Individual patient data from the study [20]: HSCT was conducted in one patient with CR | ||||
| Survival after HSCT | |||||
| Clofarabine combination therapy | < 1 year | 57.14% | 22.28% | 88.19% | [10] |
| Between 1 and 2 year | 0.00% | 0.00% | 0.00% | ||
| > 2 years | 42.86% | - | - | ||
| Nelarabine | < 1 year | 75.00% | 29.24% | 99.16% | [15, 16, 17, 18, 19] |
| Between 1 and 2 year | 25.00% | 0.84% | 70.76% | ||
| > 2 years | 0.00% | - | - | ||
| Patients lost from observations | 7.69% | 1.66% | 17.75% | [*] | |
| IDA-FLAG | Individual patient data from the study [20]; one patient survived after HSCT 2 months from transplantation. | ||||
* The proportion of patientslost from observation and alive at the latest visit was tested as the potential proportion of patients surviving longer than 2 years after HSCT.
The analysis includes adverse events influencing quality of life and generating treatment costs from the public payer perspective (febrile neutropenia, neutropenia, sepsis and bacteraemia, respiratory distress, hepatic dysfunction, central nervous system dysfunctions and mucositis). The probability of occurrence of these events is presented in Table 2.
Table 2. Adverse events
| Parameter | Mean | LCI | UCI | Source | |
| Clofarabine combination therapy | Febrile neutropenia | 8.0% | 1.0% | 21.1% | [10] |
| Neutropenia | 0.0% | - | - | [10] | |
| Sepsis and bacteraemia | 32.0% | 15.6% | 51.1% | [10] | |
| Respiratory distress | 4.0% | 0.1% | 14.2% | [10] | |
| Hepatic dysfunction and/or hyperbilirubinemia | 24.0% | 9.8% | 42.2% | [10] | |
| Central nervous system dysfunctions | 4.0% | 0.1% | 14.2% | [10] | |
| Mucositis | 12.0% | 2.7% | 27.0% | [10] | |
| Nelarabine | Central nervous system dysfunctions | 9.8% | 5.3% | 15.3% | [15] |
| IDA-FLAG | Febrile neutropenia | 92.0% | 78.9% | 99.0% | Due to lack of individual patient data from the study [20] aggregated data for Acute Myeloid Leukaemia and Acute Lymphoblastic Leukaemia wereincluded |
| Mucositis | 32.0% | 15.6% | 51.1% | ||
(*) In the analysis it was assumed that adverse events presented in the publication [10] correspond respectively to: febrile neutropenia correspond to metabolic/laboratory treatment related toxicity, sepsis and bacteraemia correspond to infections, respiratory distress correspond to lung, hepatic dysfunction and/or hyperbilirubinemia correspond to hepatic.
UTILITY DATA
The occurrence of a neoplastic disease and then oncology treatment is related with lower quality of life. Due to lack of available data on the quality of life of patients from the analysed population, values of utility coefficients were determined based on the results of a questionnaire conducted in 4 centers specialized in children’s haematology and oncology. The experts were asked to assess the quality of life on the scale 0-1 with respect to patient clinical state. The results of the survey regarding quality of life are shown in Table 3.
Table 3. Patients’ quality of life
| Patient clinical state | Quality of life | LCI | UCI | |
| Patient received palliative care | 0.26 | 0.12 | 0.43 | |
| Patient received clofarabine (or other treatment), lack of HSCT | 0.34 | 0.18 | 0.53 | |
| Patient received clofarabine (or other treatment) and HSCT and survived less than 1 year after HSCT | 0.48 | 0.31 | 0.65 | |
| Treated with clofarabine, and surviving in: | 1 year from HSCT | 0.80 | 0.38 | 0.99 |
| 2 years from HSCT | 0.85 | 0.55 | 0.99 | |
| next years | 0.88 | 0.64 | 0.99 | |
COSTS
Cost analysis was carried out from the public payer perspective, assuming that Evoltra ® was financed from public funds within the Catalogue of Chemotherapy Drugs [21].
The following cost categories important from the public payer perspective were identified:
- Clofarabine costs (Evoltra ®), cyclophosphamide and etoposide chemotherapy, nelarabine, IDA-FLAG chemotherapy (idarubicin, G-CSF, fludarabine, cytarabine), antiemetics and costs of pharmacotherapy administration;
- Costs of a hematopoietic stem cell transplantation (HSCT) and related complications;
- Costs of treating adverse events in the third-line therapy of acute lymphoblastic leukaemia;
- Costs of the patient’s condition monitoring after treatment;
- Costs of the best supporting care (BSC).
Costs are listed in Table 4.
Table 4. Costs important from the public payer perspective
| Parameter | Mean | LCI | UCI | Source | ||
| Total cost of drug (1 cycle) | Clofarabine | 5,307 PLN | 3,649 PLN | 7,271 PLN | Questionnaire study |
|
| Nelarabine | 5,265 PLN | 3,616 PLN | 7,219 PLN | |||
| IDA-FLAG | 3,120 PLN | - | - | |||
| Monitoring cost per cycle | 858 PLN | 735 PLN | 991 PLN | Questionnaire study | ||
| Best supportive care (BSC) – yearly cost | 9,251 PLN | 8,098 PLN | 10,481 PLN | Questionnaire study | ||
| Adverse events | Febrile neutropenia | 11,197 PLN | 9,595 PLN | 12,922 PLN | Questionnaire study | |
| Neutropenia | 10,443 PLN | 8,127 PLN | 13,046 PLN | |||
| Sepsis and bacteraemia | 6,266 PLN | 4,438 PLN | 8,404 PLN | |||
| Respiratory distress | 11,880 PLN | 8,207 PLN | 16,223 PLN | |||
| Hepatic dysfunction and/or hyperbilirubinemia | 3,016 PLN | 3,016 PLN | 3,016 PLN | |||
| Central nervous system dysfunctions | 1,300 PLN | 1,012 PLN | 1,623 PLN | |||
| Mucositis | 1,508 PLN | 1,406 PLN | 1,607 PLN | |||
| GVHD | 19,240 PLN | - | - | Questionnaire study | ||
| Costs of HSCT and complications of HSCT | Cost HSCT | 206,950 PLN | 184,066 PLN | 231,155 PLN | Questionnaire study | |
| Serious adverse events | 3,860 PLN | 1,991 PLN | 6,337 PLN | |||
| Infection complications treatment | 4,297 PLN | 2,427 PLN | 6,691 PLN | |||
| Non infections complications treatment | 1,400 PLN | 513 PLN | 2,725 PLN | |||
| Prevention of the disease - GVHD | 7,995 PLN | - | - | |||
| Other | 776 PLN | - | - | |||
Costs relevant for August 2012 were implemented to the analysis. While identifying medical resources used in the treatment of patients from the analysed population, the results of a survey conducted among clinical experts from medical centers specialized in children’s haematology and oncology in Poland were considered.
Discounting of health effects and costs was taken into consideration with the yearly discount rate of 3.5% for health effects and 5.0% for costs.
The cost utility threshold in Poland, according to the requirements of the Agency for Health Technology Assessment, was estimated at PLN 99,543 (three times the expected GDP per capita per year).
SENSITIVITY ANALYSIS
A deterministic and probabilistic sensitivity analysis for the results of the economic analysis were conducted. The probabilistic sensitivity analysis indicate the probability of cost effectiveness of using clofarabine in comparison to nelarabine and IDA-FLAG, with regard to the cost effectiveness threshold at a level of PLN 99,543.
Results
The analysis was based on systematic review of clinical trials. The study demonstrated that treatment with clofarabine combined with chemotherapy (cyclophosphamide and etoposide), based on the Locatelli protocol, leads to good treatment results in patients with acute (recurrent and refractory) lymphoblastic leukaemia [10]. The improvement of response rate was also demonstrated in studies for nelarabine (treatment of T-cell ALL) and IDA-FLAG protocol [15,16,17,18,19,20]. Good response rate in these patients, increases the probability for a hematopoietic stem cell transplantation leading to long term survival.
The results of costs-consequences analysis for clofarabine in combination therapy, nelarabine and IDA-FLAG in lifetime horizon and from the public payer perspective are presented in Table 5. The table summarize the results in terms of life years, quality adjusted life years and cost categories important from the public payer (National Health Fund, NHF) perspective for each analysed therapy. Clofarabine combination therapy brings 3.61 life years (LY) and 2.83 quality adjusted life years (QALY) with total cost equal to 178,120 PLN in the lifetime horizon. Nelarabine therapy brings 0.46 LY and 0.17 QALY with total cost equal to 91,404 PLN in the lifetime horizon while IDA-FLAG brings 0.64 LY and 0.28 QALY with total cost equal to 100,764 PLN.
Table 5. Costs-consequences analysis for the basic scenario, results per 1 patient
| Endpoint | Clofarabine | Nelarabine | IDA-FLAG | |
| Life years | 3.61 | 0.46 | 0.64 | |
| Quality adjusted life years | 2.83 | 0.17 | 0.28 | |
| Cost categories important from the NHF perspective | Cost of Evoltra ® |
102,611PLN | 0 PLN | 0 PLN |
| Other drugs cost | 399 PLN | 29,847 PLN | 61,261 PLN | |
| Drug administration costs | 5,730 PLN | 9,161 PLN | 4,493 PLN | |
| Monitoring costs | 927 PLN | 1,493 PLN | 1,236 PLN | |
| Adverse events costs | 4,333 PLN | 127 PLN | 10,784 PLN | |
| HSCT costs | 62,617 PLN | 48,125 PLN | 21,809 PLN | |
| BSC costs | 1,503 PLN | 2,652 PLN | 1,182 PLN | |
| Total cost from payer perspective | 178,120 PLN | 91,404 PLN | 100,764 PLN | |
| Cost effectiveness ratio (CER) | 49,341 PLN | 198,705 PLN | 157,443 PLN | |
| Cost utility ratio (CUR) | 62,940 PLN | 537,673 PLN | 359,870 PLN | |
The results of the analysis indicated that using clofarabine in combination therapy in patients from the analysed population is associated with higher costs but with better health outcomes with regard to nelarabine and IDA-FLAG.
Incremental cost effectiveness ratio (ICER) and incremental cost utility ratio (ICUR) are presented in Table 6.
Table 6. Incremental results of the basic analysis
| Difference in: | vs. nelarabine | vs. IDA-FLAG | |
| Clofarabine in combination therapy | Life years gained | 3.15 | 2.97 |
| Quality adjusted life years gained | 2.66 | 2.55 | |
| Public payer perspective cost | 86,715 PLN | 77,356 PLN | |
| ICER from public payer perspective | 27,529 PLN | 26,046 PLN | |
| ICUR from public payer perspective | 32,600 PLN | 30,336 PLN |
Gaining an additional life year resulting from the use of clofarabine in combination chemotherapy (cyclophosphamide + etoposide) in place of nelarabine and IDA-FLAG is associated with the cost of PLN 27,529 and 26,046 respectively, for the public payer in the lifetime horizon. Gaining an additional quality-adjusted life year (QALY) costs PLN 32,600 and 30,336 respectively.
As part of the deterministic sensitivity analysis for the economic analysis evaluating the use of clofarabine in combination therapy, 3 parameters most influencing results of the analysis were identified: the probability of surviving less than a year after a transplantation following the use of clofarabine in combination treatment, the probability of surviving less than a year after a transplantation following the use of nelarabine and the life expectancy of a patient who lived for over 2 years after HSCT. A change to other parameters covered in the deterministic sensitivity analysis does not result in different conclusions from the basic analysis when it comes to the cost utility assessment of using clofarabine in combination therapy instead of nelarabine or IDA-FLAG.
Table 7. Probabilistic sensitivity analysis results - the probability of cost-effectiveness of the clofarabine
| Condition | Comparator | Probability of cost-effectiveness of clofarabine in combination therapy |
| Probability that ICER | Nelarabine | 97.1% |
| IDA-FLAG | 96.5% | |
| Probability that ICUR | Nelarabine | 95.2% |
| IDA-FLAG | 95.3% |
The results of the probabilistic sensitivity analysis (Table 7) indicate that the probability of cost-effectiveness of using Evoltra ® in combination therapy, with a cost-effectiveness threshold of PLN 99,543 for a life-year gained (LYG) from the public payer perspective is equal to:
- 97.1% in comparison to nelarabine
- 96.5% in comparison to IDA-FLAG.
The probability of cost-effectiveness of using Evoltra ® in combination therapy, with a cost-effectiveness threshold of PLN 99,543 for a quality adjusted life year (QALY) gained from the public payer perspective is equal to:
- 95.2% in comparison to nelarabine
- 95.3% in comparison to IDA-FLAG.
Discussion
Acute lymphoblastic leukaemia is a rare disease which, according to the definition by the European Union, means that its morbidity does not exceed 5 cases per 10 thousand persons.
In case of drugs for rare diseases (which are defined as orphan drugs), a simplified registration procedure is applied – clofarabine was registered in a special course in the United States and the European Union on the basis of phase II studies. This is due to the fact that there is no other effective medical therapy which might be applied in patients when two previous therapies have failed or in patients who are resistant to standard chemotherapy. So far patients might have been offered IDA-FLAG chemotherapy protocol, nelarabine (in case of T-cell acute lymphoblastic leukaemia) or palliative care aimed at alleviation of disease symptoms in the last few months before death.
The limitations of the analysis are related with the character of the health problem (orphan drug used in rare disease) and restrictions of the clinical studies based on which the analysis was conducted. The studies found in the systematic review and included in the analysis were characterized by low quality resulting from lack of control group, small patient size and related with it wide range of variability of parameters. Despite the limitation of the studies, these are the most reliable and available clinical trials assessing the use of clofarabine combination therapy, nelarabine and IDA-FLAG protocol in the analysed disease. Also the limitations of the analysis result from lack of data on patient quality of life from the analysed population which made it necessary to conduct a survey.
At present, the main therapeutic goal in case of acute lymphoblastic leukaemia (ALL) in children and adolescents is to get remission of the disease and then to perform a hematopoietic stem cell transplantation which may result in recovery and long-term survival. Remission that enables HSCT for patients with refractory or recurrent acute lymphoblastic leukaemia with a poor prognosis can be obtained as a result of treatment with clofarabine, which is a significant breakthrough for these patients.
In spite of adverse events reported in clinical studies, the safety profile of Evoltra seems to be acceptable for acute lymphoblastic leukaemia, in particular among the population of patients who are often subjected to frequent and exhausting cycles of chemotherapy. It also needs to be mentioned that in 40% of patients who have been treated with clofarabine in combination with chemotherapy at a lower dose, i.e. 40 mg/m2 of body surface area (Locatelli protocol), no adverse reactions were reported [10]. For patients receiving Locatelli regimen, no case of the most severe complication of transplantation i.e. Graft-Versus-Host Disease (GvHD) was reported.
Therapy with clofarabine provides patients with refractory or recurrent acute lymphoblastic leukaemia, with two previous unsuccessful chemotherapies, a chance to become cured when there are no other therapeutic alternatives left. Therefore, although some patients treated with clofarabine may not respond to the therapy, it is recommended to use this medicinal product as an agent which may allow proceeding to the next stage of the therapy, i.e. HSCT, which is deemed to be curative therapy.
The analysed clinical trial allowed demonstrating that treatment with clofarabine combined with chemotherapy (cyclophosphamide and etoposide), based on the Locatelli protocol, leads to better treatment results in patients with acute (recurrent and refractory) lymphoblastic leukaemia [10]. This combination with clofarabine results in good response in these patients, thus increasing the probability for a hematopoietic stem cell transplantation leading to long term survival.
The analysis showed treatment with Evoltra ® of the analysed disease under Polish conditions is highly cost-effective from the public payer perspective compared with reimbursed optional therapies (nelarabine, IDA-FLAG). Gaining an additional life-year by using clofarabine in combination with chemotherapy (cyclophosphamide + etoposide) instead of nelarabine and IDA-FLAG regimen is equal to PLN 27,529 and 26,046 respectively, in lifetime horizon from the public payer perspective. Gain of an additional quality-adjusted life-year (QALY) is estimated at PLN 32,600 and 30,336 respectively. It is far below the cost effectiveness threshold of PLN 99,543. The cost-effectiveness analysis for orphan drugs such as clofarabine might be conducted on the basis of non-standard criteria and might allow accepting the cost of an additional life-year in perfect health higher than the standard cost effectiveness threshold of PLN 99,543. The triple value of GDP per capita, i.e. 99,543, for a therapeutic effect (LYG or QALY gained) is a threshold recommended by WHO in case of standard cost-effectiveness analyses which do not cover rare diseases. In United Kingdom, the cost effectiveness threshold for products which are used in rare diseases is GBP 200-300k, i.e. 10 times more than the cost effectiveness threshold for “standard” medications – ones that are not defined as orphan drugs [23].
Treatment with clofarabine (Evoltra) can considerably increase the survival of patients especially those that undergo HSCT, which in case of neoplastic condition is, apart from quality of life, a key indicator of therapy effectiveness.
Conclusions
The economic analysis demonstrated that the use of clofarabine (Evoltra) in combination with cyclophosphamide and etoposide in Poland is cost-effective when compared to nelarabine and IDA-FLAG in lifetime horizon in recurrent and refractory acute lymphoblastic leukaemia among children and adolescents, after receiving at least two prior standard lines and in patients where other options that predict a long-term response are limited.