Adverse skin reactions induced by antibiotics: clinical and economic evaluation - an eleven - year retrospective study
-
Copyright
© 2015 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Background: The aim of the study was to clinically evaluate, as well as analyse the direct costs of treating patients hospitalised due to adverse skin reactions related to antibiotics use at the Department of Dermatology of the Military Institute of Medicine, Ministry of Defence in Warsaw, during the years 2002-2012.
Method: The study was carried out in a retrospective way on a group of 164 adult patients due to adverse skin drug reactions. The analysis was based on data from patient medical records and medical orders.
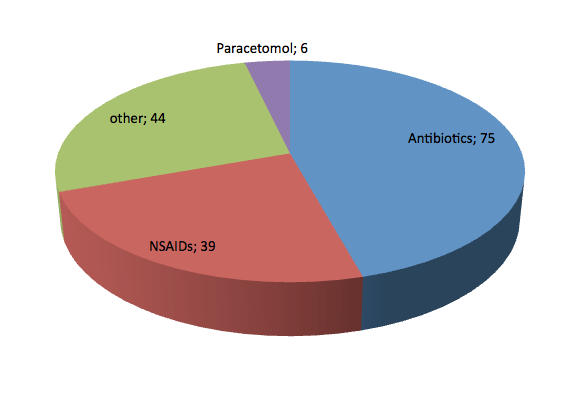
Results: The most common drugs that caused adverse skin reactions in the examined population between the 2002-2012 were antibiotics with 75 cases, in 39 cases non-steroidal anti-inflammatory drugs (NSAIDs), and in the case of paracetamol 6, in 44 cases other drugs. Amoxicillin was the most common described antibiotic (25.33%). It has been estimated that patient hospitalisation at the Department of Dermatology for adverse skin drug reaction generated costs averaging to an amount of 2784 PLN (662 EUR) per patient.
Conclusions: The most frequent form of drug-induced skin reactions, diagnosed in the study group, was the maculopapular rash - (52%). The most common drugs that caused skin lesions were antibiotics. Amoxicillin was the most common described antibiotic (25.33%). The largest average cost have been incurred due to clindamycin - 1059.91 PLN (252 EUR) and to amoxicillin - 1029.56 PLN (245 EUR). Following our analysis, treatment of skin adverse drug effects caused by antibiotics generates significant costs.
Introduction
Antibiotic-associated adverse events lead to hospitalization, and allergic reactions are the most common of these events [1]. Following the definition of the Directive 2010/84/EU of the European Parliament and the European Council on 15th of December 2010; adverse drug reactions (ADR) are noxious and unintended effects resulting not only from the authorised use of a medicinal product at normal doses, but also from medication errors and uses outside of the clinical guidelines, including the misuse and abuse of the medicinal product. The incidence of adverse drug reactions is estimated at approximately 5 – 10% of all hospitalised patients [2], out of which, 30% include adverse skin reactions (ASR) [3].
The results of these studies are of significance, can support decisions, which implement new and/or modify actual therapeutic standards. Reviewing of international publications, a growing interest in the economic component of ADRs treatment is observed in many countries [4,5]. In Poland, some clinical pharmacology centres evaluate the efficacy and costs of therapies in broad pharmacoeconomic evaluation programmes [6,7]. However during literature searches, no analyses have been identified, dedicated to adverse drug reactions, associated with skin reactions and costs of their treatment under Polish conditions. Our study attempted to estimate the costs of therapy in cases of drug-induced skin reactions under Polish conditions.
Objective
The aim of the analysis was to clinically evaluate, as well as analyse the direct costs of treating patients hospitalised due to adverse skin reactions related to antibiotics use at the Department of Dermatology of the Military Institute of Medicine, Ministry of Defence in Warsaw, during the years 2002-2012.
Methodology
It was a retrospective study, carried out in a group of 164 adult patients, hospitalised in Department of Dermatology, Military Institute of Medicine, during the years of 2002-2012 due to drug-induced skin reactions, including 57 men and 107 women. The mean age of the patients was 53.7 years. All the included patients were hospitalized due to adverse, drug-induced skin reactions, regardless of administered therapy.
Analysis
Direct costs of treatment of adverse skin drug reactions were evaluated. The analysis was based on data from the patients’ medical records and from their medical order cards. All those documents provided information on used resources, such as diagnostic tests, specialist consultations, medicinal products used for treatment and the length hospitalisation. Based on the identified resources, used in applied treatment, the costs of therapy were estimated. Due to the lack of specific data on drug costs and diagnostic tests in 2002-2010, the analysis was based on the valuation procedures of 2012. The costs of pharmacotherapy were calculated on the basis of the wholesale prices for drugs, fixed for the year 2012. The unit costs of laboratory tests, consultations and hospitalisation days were taken from the hospital’s internal pricelist, prepared by the Department of Medical Service Sales and Analysis of the Military Institute of Medicine for 2012.
Results
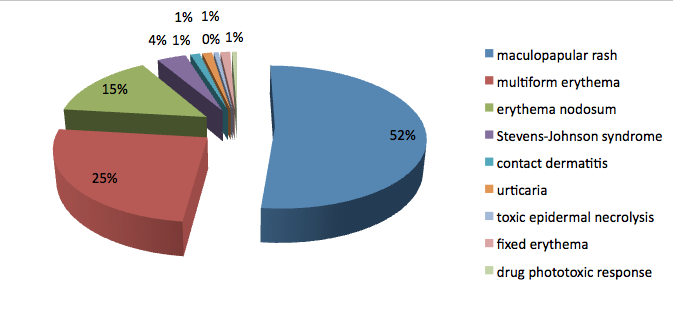
The most frequent forms of drug-induced skin reactions, diagnosed in the study group, included maculopapular rashes - (52%), multiform erythema – 25%, nodal erythema – 15% and Stevens-Johnson Syndrome (SJS) – 4%. Other causes, which accounted for 4% of cases, included contact dermatitis and allergic urticaria.
The most common drugs that caused adverse skin reactions in the analysed population were antibiotics with 75 cases, in 39 cases non-steroidal anti-inflammatory drugs (NSAIDs), and in the case of paracetamol 6, in 44 cases other drugs. Amoxicillin was the most common described antibiotic (25.33%). Cutaneous drug reactions appeared on average on day 6 (6.35) of the application of the drug. The average age of our patients was 48 years of age (47.9). Within the nonsteroidal anti-inflammatory drugs the most frequently reported changes were with the use of ibuprofen, after an average of 6 days after the administered treatment. The average age of patients was 55 years. The hospitalization for this reason, averaged 5 days (4.97).
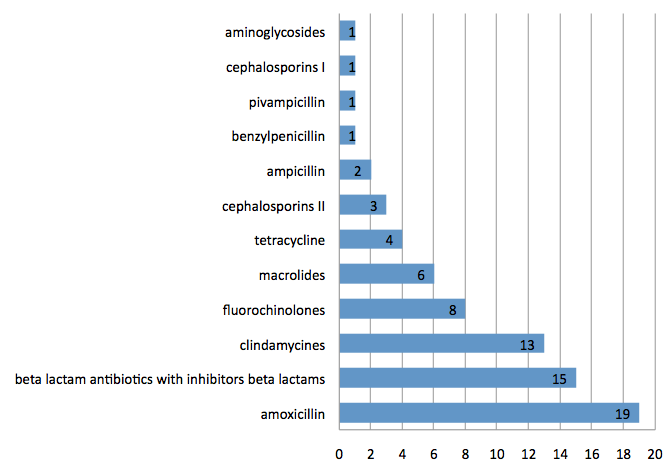
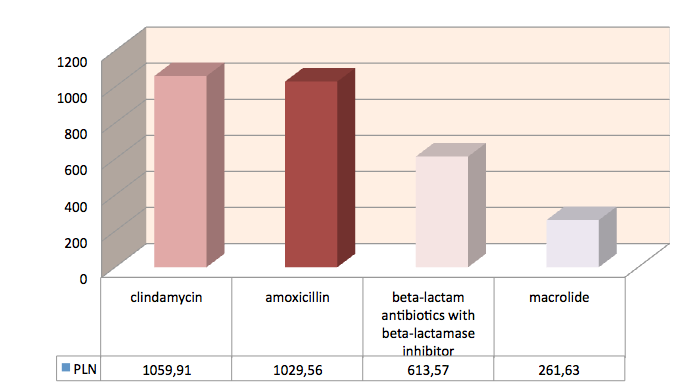
The detailed division of antibiotics is shown on the graph below.
The 23 cases were beta-lactam antibiotics, of which there were 19 cases of skin reactions after amoxicillin, two cases - ampicillin, 1 - pivampicillin, 1 - benzylpenicillin, 15 cases - beta-lactams in conjuction with inhibitors of beta-lactamases, 3 - second-generation cephalosporin and 1 case of first-generation cephalosporins. There were 32 other instances from non-beta lactam antiobiotics. Within this one case of skin reactions was due to an aminoglycoside, 8 cases were caused by fluoroquinolones, 13 cases were due to clindamycin, macrolides in 6 cases and finally 4 cases were due to tetracyclines.
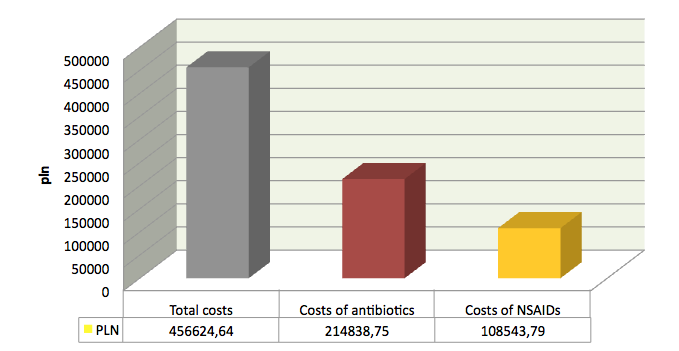
The total costs of hospitalisation in the analysed period, incurred by the Military Institute of Medicine and calculated according to the internal pricelist for the year 2012 for the analysed study group amounted to 456 624,64 PLN (108720 EUR). The average hospitalisation costs per patient was 2784 PLN (662 EUR). Analysing the direct medical costs from the medical provider’s perspective, the total costs of performed diagnostic and laboratory tests (including haematology, biochemistry, urine tests, lipid profile, x-ray examination), incurred by the hospital, were calculated, amounting to 9873,36PLN (2350 EUR). The average examination and test costs per patient were 60,22 PLN (14 EUR). The total cost of pharmacotherapy was 14753,99 PLN (3512 EUR) and the average cost of pharmacotherapy per one patient was 89,96 PLN (21 EUR). The direct costs of cutaneous drug reactions induced by antibiotics amounted to – 214 838.75 PLN (51 152 EUR), NSAIDs – 108 543 PLN (25 843 EUR).
The largest average cost was incurred due to clindamycin - 1059.91PLN (252 EUR), amoxicillin - 1029.56 PLN (245 EUR), beta-lactam antibiotics with beta-lactamase inhibitor generated a cost amounting to 613.57 PLN 146 EUR) and macrolide 261.63 PLN (62 EUR).
Discussion
In the era of liberal use of antibiotics on the market, the issue of drug-induced reactions is at an all time high. Patients with such changes will require treatment in the hospital environment. The diagnostics and therapy of the hospitalised patients require evaluations of these costs. Accordingly, we carried out an analysis of direct medical costs, incurred by the medical service provider in a group of 164 patients, hospitalised at the Department of Dermatology of the Military Institute of Medicine for antibiotics-induce adverse skin reactions in an eleven-year retrospective study.
The most common drugs that were the cause of cutaneous drug reactions in the analyzed study were still beta-lactam antibiotics and NSAIDs [8-11].
We did a clinical evaluation of drug-induced adverse skin reactions and an evaluation of the costs of their treatment.
Our study of the clinical data analysis can be compared with epidemiological analysis conducted in 2008 in the hospital of Pomeranian area. The patients with cutaneous drug reactions (386) were hospitalized from January 1996 to December 2006. The analyzed group was 4.25% of all hospital admissions over the 11 analyzed years. The Drugs that most frequently triggered cutaneous drug reactions (37.6%) were non-steroidal anti-inflammatory drugs. Amino-penicillin antibiotics, especially amoxicillin resulted to be the cause of skin reactions in 25.8% of cases. The most common figures that were reported: erythematous rash and maculopapular - rash (42%), acute urticaria together with Quincke edema (39.1%) and contact dermatitis (8%). Also diagnosed was erythema multiforme (7.3%). In addition, two instances of severe: toxic epidermal necrolysis and Stevens-Johnson syndrome (TEN and SJS). The maculopapular rash were usually caused by beta-lactam antibiotics with a beta-lactamase inhibitor (48.6%). Amoxicillin (21.4%) and carbamazepine (17.8%) were the cause of the reported cases of erythema multiforme [12].
Other examples of clinical analysis are the described below and performed in Thailand, India and Singapore.
In Thailand (Siriraj Hospital, Bangkok, Thailand) in the period from January 2002 to February 2012, in a population of elderly patients a retrospective analysis of cutaneous drug reactions was carried out. The results were similar and allowed for a comparison. The study included 400 medical records of history in patients above 60 years of age. Women accounted for 53% of all patients. Mean age was 73.6 years (62-96 years). The most common drug reactions included skin rash maculo - papular, which accounted for 65%. Most often they were caused by antibiotics (42.8%) and non-steroidal anti-inflammatory drugs (9,5%) in all the diseases described in 16.5% of patients, severe cutaneous drug reactions [13].
In another prospective study conducted in a large hospital in India in IGGMC and H analysing patients’ data about cutaneous drug reaction we observed similarity of epidemiology in relation to our analysis. The analysis was conducted from 1st June 2005 to 31 May 2009. The group consisted of 2,693 patients, over 872 patients (33.4%) had cutaneous drug reactions, including 560 men and 312 women. The most common medicines, which caused 55.5% were antibiotics, glicocorticoids - 18.56% and NSAIDs - 12.61%. The most often observed ASR was maculopapular rash 37.73%, 17.2% fixed erythema and urticaria 14.56%. Attention needs to be drawn to the use of antibiotics and NSAIDs, because they accounted for at least one severe cutaneous drug reaction [14].
Severe cutaneous adverse reactions (SCAR), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS) can be potentially life threatening.
Another study, performed in Singapore analysed data retrospectively for the period from January 2007 to December 2011. They analysed a total of 42 patients. The average age was 51.8 years (11-94 years). The gender distribution was equal (21). Most reported reactions occurred after exposure to antibiotics 50%, allopurinol - 14.3%, NSAID - 9.5%. SJS was observed most frequently (54.8%). Changes in the skin after exposure to antibiotics was observed after an average of nine days. The symptoms of SJS were observed on average on the 22nd day. The band overlap SJS-TEN lesions were observed after only 5 days. In 38.1% of patients with systemic complications occurred. The most common were hypertransaminesemia (37.5%), disorders of the gastrointestinal tract and diarrhea (25%). In one case (overlap syndrome SJS-TEN complicated by pneumonia) lead to death [15].
In relation to the cost analysis we performed we were unable to obtain information about other direct costs of the treatment of cutaneous drug reactions caused by antibiotics in the available literature. A lot of reports dealt in terms of the cost of treatment of systemic side effects, but not the cutaenous.
Our analysis estimated that hospitalization in the Department of Dermatology Military Institute of Medicine due to drug reactions accounted for an average amount of 2784.30 PLN (662 EUR, 921 USD). Because of the absence of other cost analysis regarding cutaneous drug reactions in the Polish literature in order to enable the comparison with other published international analysis the costs results have been converted to EUR and USD (1 EUR - 4.20 PLN, 1 USD- 3.02 PLN).
In May 2013, a study on the costs of the treatment of cutaneous drug reactions in hospitalized patients in Taiwan was published. The data was collected by the medical center Chung Gung Memorial Hospital in northern Taiwan. In the analyzed group from 2005 to 2008 there were observed 700 patients with cutaneous drug reactions, including 370 men and 330 women. In the control group there were 3365 patients without cutaneous drug reactions, including 1785 men and 1580 women. Their mean age was 49 years and 48 years for the control group, the average length of hospital stay amounted to 18 days for cutaneous drug reactions, and 7 days for the control group. The most common reactions were skin maculopapular rash 89.3%, and Stevens - Johnson 3.3%. The most common drugs that caused these reported reactions were antibiotics (57%). Others included antiepileptic drugs (9.8%), antipyretics (9.7%). Medical costs were estimated at an average of 916 USD. Compared with the control group (cost medical 318 USD) the costs are 2.5 times higher. In estimating costs of treatment, of critical importance was the length of hospitalizations and severity of condition of patients [16].
In 2014, an interesting publication on a retrospective analysis of ADR in the period from 1 January 2008 to 31 December 2012 in China appeared. All cases have been reported to the ADR Center database. The following data was obtained from the medical records of patients with the ADR. All cases of ADR were divided into two groups depending on the severity of adverse drug reactions. Group A included cases that resolve after cessation of treatment, that did not require further hospital intervention. Serious drug reactions and complications associated with them (including: death or life-threatening conditions leading to permanent disability) were included into group B. The study included 2,739 cases of drug-induced adverse reactions. The mean age of the patients was 48.51 ± 19.84 years (1 day of life - 99 years). Women accounted for 52.28% of all patients, 47.72% were men. Antibiotics were the most common cause of ADRs (34.9%), mainly levofloxacin (7%). Most reported cutaneous drug reactions (31.69%), systemic ADR (30.56%) and gastrointestinal disorders (17.71%). Mild to moderate reactions were respectively 59.47% and 39.03%, only in 1.5% reported severe ADR. Not all types of diseases were analyzed in terms of epidemiology and cost. The direct and indirect costs of hospitalization in ADR were divided into the above-mentioned group A and B. The costs in group A were 43604.66 EUR, average 1616.18 EUR in group B was much higher in terms of the average cost 34,724.80EUR, medium 846.85 EUR [17].
Another article presents an analysis of the incidence of adverse reactions with the medications and the costs of their treatment in a hospital in Taiwan. This is a prospective study conducted during the period from 1st January 2002 to 31st December 2004. The study enrolled 564 patients from 142,295 patients hospitalized for ADR. Men constituted the majority in the study - 56.4%, the average age of the patients was 66 years. The most common cause of ADR were by antibiotics (38.8%), analgesics 11% and 9.9% cardiac drugs. The most common were cutaneous drug reactions, which accounted for 52.5% of all ADR, the maculopapular rash accounted for 30.5%, 6.6% erythema of site administration. Hematologic drug reactions were 10.8%. In this study, the average cost of hospitalizations associated with ADR was US 3489. These being the total cost of all the ADR, including those patients with cardiovascular complications [18].
In the available literature there are a lot of articles on pharmacoeconomic analysis related to treatment of other skin diseases which may impact on the economic system of hospitals [19,20].
Thanks to such work, an analysis of the cost of the treatment can be carried out, support the decision about the choice of the medication that is the best for the patient and at the same time assess the burden of the hospital budget.
There are a lot of evaluations of studies also in terms of the quality of life of patients, the effectiveness of the treatment, costs of disease entities [21-25].
However, each patient is a separate case, even the same disease may require a longer or shorter stay in the hospital, or cheaper or more expensive laboratory diagnostics. These costs shall be borne by the provider in such a situation.
Therefore, the analysis of costs is extremely important when planning the budget, when implementing new billing systems of medical services and improving already existing ones. Such type of studies can be extremely useful management tools for departments through to the main hospital authorities.
Unfortunately, we did not find similar publications related to the cost of the treatment of cutaneous drug reactions caused by antibiotics within the available literature, which made it difficult to compare our results with other Polish departments of dermatology.
Conclusions
The most frequent forms of drug-induced skin reactions, diagnosed in the study group, was the maculopapular rash (52%). The most common drugs that caused skin lesions were antibiotics. Amoxicillin was the most common described antibiotic (25.33%). The largest average cost have been incurred due to clindamycin - 1059.91 PLN (252 EUR) and to amoxicillin - 1029.56 PLN (245 EUR). Following our analysis, treatment of skin adverse drug effects caused by antibiotics generates significant costs.
- Shehab N., Patel PR., Srinivasan A., Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008 Sep 15; 47(6):735-43
- Stausberg J., Hasford J. Drug-related admissions and hospital-acquired adverse drug events in Germany: a longitudinal analysis from 2003 to 2007 of ICD-10-coded routine data. Health Services Research 2011; 11:134
- Burbach GJ., Zuberbier T. Diagnosis of drug-induced skin reactions: a future role for computer-aided systems ? Curr Opin Allergy Clin Immunol. 2011 OCT; 11(5):451-6
- Rottenkolber D., Schmiedl S., Rottenkolber M., Farker M., Saljé K., Mueller S., et al. Adverse drug reactions in Germany: direct costs of internal medicine hospitalizations. Pharmacoepidemiology and drug safety 2011; 20: 626–634
- Pattanaik S., Dhamija P., Malhotra S., Sharma N., Pandhi P. Evaluation of cost of treatment of drug-related events in a tertiary care public sector hospital in Northern India: a prospective study. Br J Clin Pharmacol. 2009 Mar; 67(3):363-9
- Więczkowska H., Bryl W., Hoffmann K. Costs of diagnosis and treatment of hypertension in the department of internal diseases, Forum Zaburzeń Metabolicznych 2011; tom 2, nr 3: 177–183
- Kusowska J. Economic evaluation of treatment of community acquired respiratory tract infections, Farmakoekonomika 2001; 1
- Grubska-Suchanek E.: Skórne odczyny polekowe. Służba Zdrowia, 2001; 17: 3012-3017
- Naldi L1, Crotti S. Epidemiology of cutaneous drug-induced reactions. Naldi L1, Crotti S. G Ital Dermatol Venereol. 2014 Apr; 149(2):207-18
- Doña I., Barrionuevo E., Blanca-Lopez N., Torres MJ., Fernandez TD., Mayorga C., Canto G., Blanca M. Trends in hypersensitivity drug reactions: more drugs, more response patterns, more heterogeneity. J Investig Allergol Clin Immunol. 2014; 24(3):143-53
- Rodriguez-Pena R1, Antunez C., Martin E., Blanca-Lopez N., Mayorga C., Torres MJ. Allergic reactions to beta-lactams; Expert Opin Drug Saf. 2006 Jan; 5(1):31-48
- Kacalek – Rzepka A., Klimowicz A., Bielecka – Grzela S., Załuga E., Maleszka R., Fabiańczyk H. Retrospektywna analiza niepożądanych skórnych reakcji polekowych u pacjentów hospitalizowanych w Klinice Chorób Skórnych i Wenerycznych Pomorskiej Akademii Medycznej w latach 1996-2006
- Tuchinda P., Chularojanamontri L., Sukakul T., Thanomkitti K., Nitayavardhana S., Jongjarearnprasert K., Uthaitas P., Kulthanan P., Cutaneous Adverse Drug Reactions in the Elderly: a Retrospective Analysis in Thailand
- Hiware S., Shrivastava M., Mishra D., Mukhi J., Puppalwar G. Evaluation of Cutaneous Drug Reactions in Patients Visiting Out Patient Departments of Indira Gandhi Government Medical College and Hospital (IGGMC and H), Nagpur
- Peiqi Su, MBBS, BSC, MRCP, and Chen Wee Derrick Aw, MBBS, MRCP, FAMS Severe cutaneous adverse reactions in a local hospital setting: a 5-year retrospective study International Journal of Dermatology, November 2014; Vol. 53, Issue 11: 1339–1345
- Liao PJ., Shih CP., Mao CT., Deng ST., Hsieh MC., Hsu KH. The cutaneous adverse drug reactions: risk factors, prognosis and economic impacts. Int J Clin Pract. 2013 Jun; 67(6):576-84
- Qing-Ping S., Xiao-Dong J., Feng D., Yan L., Mei-ling Y., Jin-Xiu Z., Shu-Qiang Z. Consequences, measurement, and evaluation of the costs associated with adverse drug reactions among hospitalized patients in China BMC Health Serv Res. 2014; 14: 73
- Agnes LF. Chan, MAMM; Haw Yu Lee, MD Chi-Hou Ho, MS; Thau-Ming Chain, PhD; and Shun Jin Lin, PhD , Cost Evaluation of Adverse Drug Reactions in Hospitalized Patients in Taiwan: A Prospective, Descriptive, Observational Study Current Therapeutic Research (Impact Factor: 0.45). 01/2008; 69(2):118-129
- Guy GP. Jr1, Ekwueme DU., Tangka FK., Richardson LC., Melanoma treatment costs: a systematic review of the literature, 1990-2011; Am J Prev Med. 2012 Nov; 43(5):537-45
- Clarke AE., Urowitz MB., Monga N., Hanly JG. Costs associated with severe and non-severe SLE in Canada. Arthritis Care Res (Hoboken). 2014 Sep 3
- Palmieri V., Baldi C., Di Blasi PE., Citro R., Di Lorenzo E., Bellino E., Preziuso F., Ranaudo C., Sauro R., Rosato G. Impact of DRG Billing System on Health Budget Consumption in Percutaneous Treatment of Mitral Valve Regurgitation in Heart Failure; J Med Econ. 2014 Oct 28: 1-20
- Lahue BJ., Pyenson B., Iwasaki K., Blumen HE., Forray S., Rothschild JM. National burden of preventable adverse drug events associated with inpatient injectable medications: healthcare and medical professional liability costs. Am Health Drug Benefits. 2012 Nov; 5(7):1-10
- Franz D., Schemmann F., Selter DD., Auhuber T., Gehweiler D., Roeder N., Siebert H., Mahlke L. Quality of case allocation of orthopedics and trauma surgery in the 2004 and 2014 versions of the German DRG system: An interim assessment of the development process; Unfallchirurg. 2014 Oct; 117(10):946-56
- Schreyögg J., Stargardt T., Tiemann O., Busse R. Methods to determine reimbursement rates for diagnosis related groups (DRG): A comparison of nine European countries; Health Care Manage Science, 2006; tom 9: 215-223
- Schreyögg J., Tiemann O., Busse R. Cost accounting to determine prices: How well do prices reflect costs in the German DRG-syst em?; Health Care Manage Science, 2006; tom 9: 269-279