Cancer immunotherapy in second-line treatment of non-small cell lung cancer – is there a need to change the approach to the assessment of clinical benefits?
-
Copyright
© 2018 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
| Name | Affiliation | |
|---|---|---|
Anna Panasiuk |
Aestimo s.c., Kraków, Poland |
|
Rafał Wójcik |
Aestimo s.c., Kraków, Poland |
|
Małgorzata Budasz-Świderska |
Roche Polska Sp. z o.o., Warszawa, Poland |
|
Marcin Kaczor |
Aestimo s.c., Kraków, PolandUniwersytet Jagielloński Collegium Medicum, Kraków, Poland |
Background: The mechanism of action distinctive for immunotherapy leads to the specific pattern of tumour response, different form the one observed with cytotoxic therapy. To enable a complete assessment of immunotherapeutic agents modified criteria for the evaluation of antitumour responses were developed, mainly on the basis of the ipilimumab trials in melanoma.
Methods: We conducted a review of RCTs that assessed PD-1/PD-L1 checkpoint inhibitors in previously treated patients with NSCLC, to describe the overall survival results in comparison to the progression-free survival outcomes, as assessed with conventional RECIST and/or with modified criteria of tumour response.
Results: Five RCTs were eligible. The studies were heterogeneous with respect to population characteristics and other methodological features. All of the trials showed significant OS improvement in the immunotherapy arms, with the results in the histology and PD-L1-expression subgroups consistent with ITT analyses. The OS gain was not accompanied with the evidence of PFS prolongation. The use of the modified criteria resulted in prolonged median PFS estimates in the immunotherapy-treated patients.
Conclusion: Our results in second-line NSCLC population are in line with the previous findings, that the conventional RECIST criteria do not fully capture the benefit of the immunotherapy. In the routine practice the premature diagnosis of a progressive disease may result in treatment withdrawal in patients that might benefit from the continuation of the immunotherapy. Further research and the agreement on the relevant modified criteria are needed for the use in the future immunotherapy clinical trials and to inform real-world clinical decisions.
Introduction
Immunotherapy is a highly innovative strategy for the cancer treatment, showing promising results in a variety of neoplasms, including those with very poor prognosis. From the beginning of cancer immunotherapies development it was noted that the distinctive mechanism of action leads to the specific pattern of tumour response, different form the one observed with cytotoxic therapy. The measurable clinical response may appear later, even after initial tumour burden increase (a phenomenon called “pseudoprogression” or “tumour flare”), and the clinical benefit may be represented by durable stable disease [1]. That observations led to the conclusion that conventional Response Evaluation Criteria In Solid Tumours (RECIST) [2, 3] or World Health Organization (WHO) [4, 5] criteria might not be suitable for the assessment of cancer immunotherapies [1, 6 – 8]. In early phases of drug-therapy development the inadequate assessment, based on underestimated response rates or overestimated relapse rates, may lead to the abandonment of potentially efficient treatments. At the latest stages, represented by phase 3, pre-approval clinical trials, this specific pattern of response may result with a confusing discordance between the response-related results, such as response rate or progression-free survival (PFS), and the survival-based results (mainly overall survival; OS).
To provide a basis for a complete assessment of novel immunotherapeutic agents, modified criteria for the evaluation of antitumour responses were developed. Immune-Related Response Criteria, for the Evaluation of Immune Therapy Activity in Solid Tumors (irRC) were published in 2009 as a new tool for clinical investigation of immune therapy in cancer patients and a potential guidance for clinical care [9]. The key novelty of the irRC was the incorporation of measurable new lesions into “total tumour burden” and comparison of this domain to baseline measurements before and after WHO
progressive disease (PD), but not after confirmed irPD [9]. The Immune-Related Response Criteria were developed mainly on the basis of the clinical data for CTLA-4 inhibitor ipilimumab in an advanced melanoma population [9]. Further modifications proposed by Nishino (i.e. the use of unidimensional tumour measurements used in RECIST v1.1 rather than bidimensional measurements used in WHO criteria – irRECIST1.1 criteria), were also based on the ipilimumab results in the advanced melanoma population [10 – 12]. In spite of the inclusion of many other observations in the concept of irRC, including other types of immunotherapies and patients [9], the extensive validation of the modified criteria in non-melanoma trials was advocated and has been already undertaken [13]. The issues with application of the conventional tumour assessment criteria to describe clinical benefit of the new immunotherapies are also acknowledged in the clinical guidelines [14, 15], as well as is the need for further studies that will allow for more complete description of different response profiles [14]. In fact, despite the development of the modified assessment tools, tailored to the specific dynamics of the immune response, the response-based endpoints in the clinical trials investigating the new cancer immunotherapies still rely primarily (i.e. for a purpose of primary or secondary endpoints assessment) on the conventional RECIST criteria [8, 16].
The last years brought several approvals of new, promising immunotherapies for the patients with advanced non-small-cell lung cancer (NSCLC) by the European Medicines Agency (EMA) and the U. S. Food and Drug Administration (FDA). The approved agents belong to the class of the immune checkpoint inhibitors, targeting programmed death 1 protein (PD-1)/programmed cell death ligand 1 (PD-L1) checkpoint pathway (anti-PD-1/PD-L1 antibodies). Programmed death 1 receptors are expressed on activated cytotoxic T-cells. Both PD-1 and its ligand, PD-L1, play a key role in the formation of tumour microenvironment, contributing to tumour generation and growth. Thus, blockading of the PD-1/PD-L1 pathway has a potential to reverse the tumour microenvironment and enhance the endogenous antitumor immune responses [17, 18, 19]. Currently there is one anti-PD-L1 and two anti-PD-1 antibodies authorised by EMA for the treatment of incurable locally-advanced or metastatic non-small NSCLC (advanced NSCLC). A PD-1 inhibitor nivolumab (brand name: Opdivo) and a PD-L1 inhibitor atezolizumab (brand name: Tecentriq, approved in 2017) are indicated for the treatment of advanced NSCLC after prior chemotherapy [20, 21]. A PD-1 inhibitor pembrolizumab (brand name: Keytruda) is indicated also for the first-line treatment, but it should be used only in patients whose tumours express PD-L1 (with a ≥1% or ≥50% tumour proportion score, in previously treated or untreated patients, respectively) [22]. All of those therapies have already been recommended in the most up-to-date (2017) clinical guidelines [15, 23].
We conducted a review of randomised controlled trials (RCTs) that assessed PD-1/PD-L1 checkpoint inhibitors, atezolizumab, nivolumab or pembrolizumab, in previously treated patients with NSCLC. Our objective was to describe the overall survival (OS) results in comparison to the evaluation of a response-based endpoint, namely, progression-free survival (PFS), as assessed with classical RECIST criteria and/or with modified criteria of tumour response, if available.
Materials and methods
The Cochrane Library, PubMED and Embase were searched in November 2017 using combined search terms for non-small-cell lung cancer, immune checkpoint inhibitors and random allocation of patients. We also searched for relevant meeting abstracts and posters in The European Society for Medical Oncology (ESMO), The American Society of Clinical Oncology (ASCO), and The International Association for the Study of Lung Cancer (IASLC)/World Conference on Lung Cancer (WCLC) proceedings. The studies meeting the following criteria were included: (i) RCTs comparing atezolizumab, nivolumab or pembrolizumab, in EMA-approved doses, to standard chemotherapy in previously treated patients with advanced NSCLC, defined as incurable locally advanced, metastatic or recurrent disease; (ii) reporting hazard ratios (HRs) for OS or PFS; (iii) published in English or Polish. Unpublished trials were not eligible, but we included meeting abstracts and posters containing OS or PFS results obtained in prolonged follow-up or additional data on PFS assessment according to modified tumour response criteria. The relevant marketing authorisation holders’ (MAH) submissions to The National Institute for Health and Care Excellence (NICE) were additionally checked for otherwise unpublished results in relevant patient subgroups or PFS assessment based on modified criteria.
We extracted OS and PFS results for intention-to-treat (ITT) populations and for the subgroups defined with respect to (i) PD-L1 expression (PD-L1-“positive” and “negative” subgroups) and (ii) histological subtype of NSCLC (non-squamous and squamous carcinomas). We did not attempt to statistically pool OS or PFS results of included studies. The key characteristics of the study design, treatments and patients were extracted to explore for the potential reasons of possible between-study heterogeneity in the results.
Results
Characteristics of the included studies
Five RCTs were eligible for the review: two trials investigated atezolizumab (POPLAR [24 – 27] and OAK [28, 29]), two – nivolumab (CheckMate 017 [30 – 33], CheckMate 057 [31 – 34] and the results in the pooled population of those two trials [35]) and one – pembrolizumab (Keynote-010 [36 – 38]). The control treatment was standard docetaxel chemotherapy in each case, while the studies were heterogeneous with respect to other important methodological features and population characteristics (Table 1.).
Table 1. Main characteristics of the studies included in the review.
|
Study |
Treatments |
Patients |
||||||||
|
Investigational immunotherapy |
Post-PD treatment* |
Control CTH |
Cross-over allowed?** |
Eligibility criteria for previous treatment |
Number of prior systemic therapies*** |
Histology subtype |
PD-L1 expression on <1% of TC§§ |
EGFR mutation-positive† |
ECOG status |
|
|
POPLAR [24] phase 2
|
ATEZO 1200 mg q3w N = 144 |
yes, 40% [26] |
DOC 75 mg/m2 q3w N = 143 |
no, but 1,4% received ATEZO in subsequent lines††† |
≥1 prior PLT-containing regimen |
1: 66% 2: 34% |
SQ: 34% NSQ: 66% |
<1%: 43% ≥1%: 38% |
11% (N = 166) |
0: 32% 1: 67% N/A: 1% |
|
OAK [28] phase 3 |
ATEZO 1200 mg q3w N = 425 |
yes, 40% |
DOC 75 mg/m2 q3w N = 425 |
no |
≥1 prior PLT-containing regimen, ≤2 prior lines of CTH |
1: 75% 2: 25% |
SQ: 26% NSQ: 74% |
<1%: 55% ≥1%: 45% (N = 400)§§§ [29] |
12% (N = 713) |
0: 37% 1: 63% |
|
CheckMate 017 [30, 35] phase 3 |
NIVO 3 mg/kg q2w N = 135 |
yes, 21% |
DOC 75 mg/m2 q3w N = 137 |
only after completion of the primary analysis; 4% crossed‡ |
1prior PLT-containing regimen |
1: 100% 2: <1% |
SQ: 100% NSQ: 0% |
<1%: 39% ≥1%: 44% NE: 17% |
N/A |
0: 24% 1: 76% N/A: 1% |
|
CheckMate 057 [34, 35] phase 3 |
NIVO 3 mg/kg q2w N = 292 |
yes, 24% |
DOC 75 mg/m2 q3w N = 290 |
only after completion of the primary analysis; 6% crossed‡ |
1prior PLT-containing regimen |
1: 89% 2: 11% Other£: <1% |
SQ: 0% NSQ: 100% |
<1%: 36% ≥1%: 42% NE: 22% |
19% (N = 422) |
0: 31% 1: 69% 2: <1% |
|
Keynote-010 [36] phase 2/3 |
PEMBRO 2 mg/kg q3w†† N = 344 |
no, but the treatment decisions were irRC-guided‡‡ |
DOC 75 mg/m2 q3w N = 343 |
no, <1% received PEMBRO in subsequent lines‡‡‡ |
≥1 prior PLT-containing regimen |
1: 70% 2: 21% ≥3: 8% Other§: 1% N/A: <1% |
SQ: 21% NSQ: 70% Other: 3% N/A: 7% |
<1%: 0% ≥1%: 100% |
8% (N = 641) |
0: 33% 1: 66% 2: 1% 3: <1% N/A: <1% |
* only in PD1/PD-L1 treatment groups; ** only in control (chemotherapy) groups; *** systemic therapies for advanced disease; † proportion of EGFR mutation-positive patients in a group with known EGFR mutation status (N); †† pembrolizumab 10 mg/kg arms was not included (not authorised dose); ††† at data cut-off for primary analysis (minimum follow-up of 13 months); ‡ at data cut-off for updated analysis (minimum follow-up of 2 years); ‡‡ after the first documentation of PD it was at the discretion of the investigator to keep a clinically stable subject on trial treatment or to stop trial treatment until repeat imaging performed later confirms progression (Subjects that are deemed clinically unstable are not required to have repeat imaging for confirmation); ‡‡‡ at data cut-off for primary analysis (median follow-up of 13 months); § prior systemic therapy was adjuvant or neo-adjuvant treatment; §§ numbers and percentages of patients with PD-L1 expression on tumour cells was given in the table for each study to allow inter-trial comparisons of the samples, but note that PD-L1expression-subgroups in POPLAR and OAK trials were defined on the basis of PD-L1 expression on both tumour cells and tumour-infiltrating immune cells; §§§ data available only for a subset of patients (biomarker-evaluable population, evaluated post-hoc with 22C3 IHC assay [29])
Abbreviations: ATEZO - atezolizumab ; CTH – cytotoxic chemotherapy treatment; DOC - docetaxel; ECOG - Eastern Cooperative Oncology Group performance-status scores; irRC - Immune-Related Response Criteria; N/A – not available (unknown); NE – not evaluable; NIVO - nivolumab; NSQ – non-squamous; PD – progressive disease; PEMBRO - pembrolizumab; PLT – platinum; SQ – squamous; TC – tumour cells
In the atezolizumab and nivolumab trials the anti-PD-1/PD-L1 treatment could have been continued beyond “classical” disease progression. At cut-off points for primary data analyses about 20% of patients continued nivolumab therapy in CheckMate trials, while in both atezolizumab studies the continuation rates were two times higher (40%). In pembrolizumab trial both study treatments were provided until the confirmed disease progression, but the treatment decisions in Keynote-010 were informed by modified response criteria (irRC) and the percentage of patients that continued immunotherapy beyond “classical” progression was not reported. In the atezolizumab and pembrolizumab trials post-progression cross-over to investigational treatment was prohibited, while the protocols of both nivolumab studies allowed for switching after completion of the primary analyses, what resulted with higher rates of patients that actually switched. Even though the cross-over rates to studied PD-1/PD-L1 inhibitors did not reached 10% in any of the trials, patients could have received other investigational immunotherapies during subsequent lines of therapy.
The eligibility criteria for previous treatment lines differed between studies. Atezolizumab was investigated as a second- or third-line therapy, with more than 2/3 of the samples receiving PD-L1 antibody as a second-line therapy, pembrolizumab trial enrolled also patients pre-treated with more than 3 previous lines of systemic treatment (70% treated in 2nd line), while nivolumab trials enrolled for second-line treatment only (eventually 90% of Checkmate 057 and 100% of CheckMate 017 samples received nivolumab as a 2nd line). With regard to histology subtypes, atezolizumab and pembrolizumab studies had mixed samples of patients with squamous and non-squamous carcinomas, while nivolumab trials enrolled separately patients with squamous NSCLC to CheckMate 017 or with non-squamous NSCLC – to CheckMate 057 trial. However, the pooled population of the nivolumab trials was very similar to the remaining samples in the proportion of each histology, with about 70% of patients with a non-squamous subtype. Patients showing very low or undetectable PD-L1 ligand expression (<1%) on tumour cells (TC) were not enrolled to the pembrolizumab trial, while the remaining studies included patients unselected for this characteristic. In each study the expression of PD-L1 ligand on TC was assessed to define PD-L1 expression level subgroups, but only in the atezolizumab trials it was used in a conjunction with PD-L1 expression on tumour-infiltrating immune cells (IC). The proportion of patients with PD-L1 expression on <1% of TC was about 40-50% of patients in atezolizumab and nivolumab samples and it was slightly higher in atezolizumab trials. Between-study comparisons may not be valid though, as for each antibody the studies used different diagnostic tests, staining platforms and protocols for the quantification of PD-L1 expression. Nivolumab trials reported that about 20% of patients were not evaluable for the assessment, while atezolizumab trials reported PD-L1-expression status for almost all of the included patients, what suggests significant differences in the evaluation method and/or data analysis. The proportion of patients with epidermal growth factor receptor (EGFR) mutation varied from 8% of patients with known mutation status in Keynote-010 to 19% in CheckMate 057 trial. The samples consisted of about 65-75% of patients with The Eastern Cooperative Oncology Group (ECOG) 1 score, with the highest proportion of this performance status in the CheckMate 017 study.
Overall survival in the ITT population was the primary or co-primary endpoint in all of the included RCTs. Progression-free survival per RECIST v1.1. was assessed as the secondary or co-primary endpoint. Additional, exploratory PFS assessment with modified criteria of tumour response, was provided in only two trials: POPLAR (using imRECIST criteria, developed by MAH) and Keynote-010 (irRC). The studies differed also with respect to the time of first assessment of response and in some other details of response assessment timelines, as described in Table 2.
Table 2. Response/progression assessment in the studies included in the review.
|
Study |
Immunotherapy |
Response assessment criteria for PFS |
Post-baseline response assessment timelines |
|
|
RECIST (assessor) |
modified criteria |
|||
|
POPLAR [24] |
ATEZO |
yes, v1.1 (INV) |
yes, imRECIST (exploratory) |
every 6 weeks for 36 weeks after randomisation, and every 9 weeks thereafter, until disease progression; for patients who continued ATEZO beyond progression, assessments continued until discontinuation |
|
OAK [28] |
ATEZO |
yes, v1.1 (INV) |
no |
every 6 weeks for 36 weeks after randomisation, and every 9 weeks thereafter, until disease progression; for patients who continued ATEZO beyond progression, assessments continued until discontinuation |
|
CheckMate 017 [30, 35] |
NIVO |
yes, v1.1 (INV) |
no |
at week 9 and every 6 weeks thereafter; for patients who continued NIVO beyond progression, the best overall response were determined based on response designations recorded up to the time of the initial RECIST 1.1-defined progression |
|
CheckMate 057 [34, 35] |
NIVO |
yes, v1.1 (INV) |
no |
at week 9 and every 6 weeks thereafter; for patients who continued NIVO beyond progression, the best overall response were determined based on response designations recorded up to the time of the initial RECIST 1.1-defined progression |
|
Keynote-010 [36] |
PEMBRO |
yes, v1.1 (IRR) |
yes, irRC (exploratory) |
every 9 weeks, until the start of a new antineoplastic therapy, documented disease progression, or death |
Abbreviations: ATEZO – atezolizumab; imRECIST - immune-modified RECIST criteria; INV – the occurrence of disease progression was determined by the investigator; IRR - the occurrence of disease progression was determined by the independent radiologists’ review; irRC - immune-related response criteria; NIVO - nivolumab; PEMBRO - pembrolizumab; PFS – progression-free survival; RECIST - Response Evaluation Criteria In Solid Tumours
Overall survival results and progression-free survival per RECIST 1.1
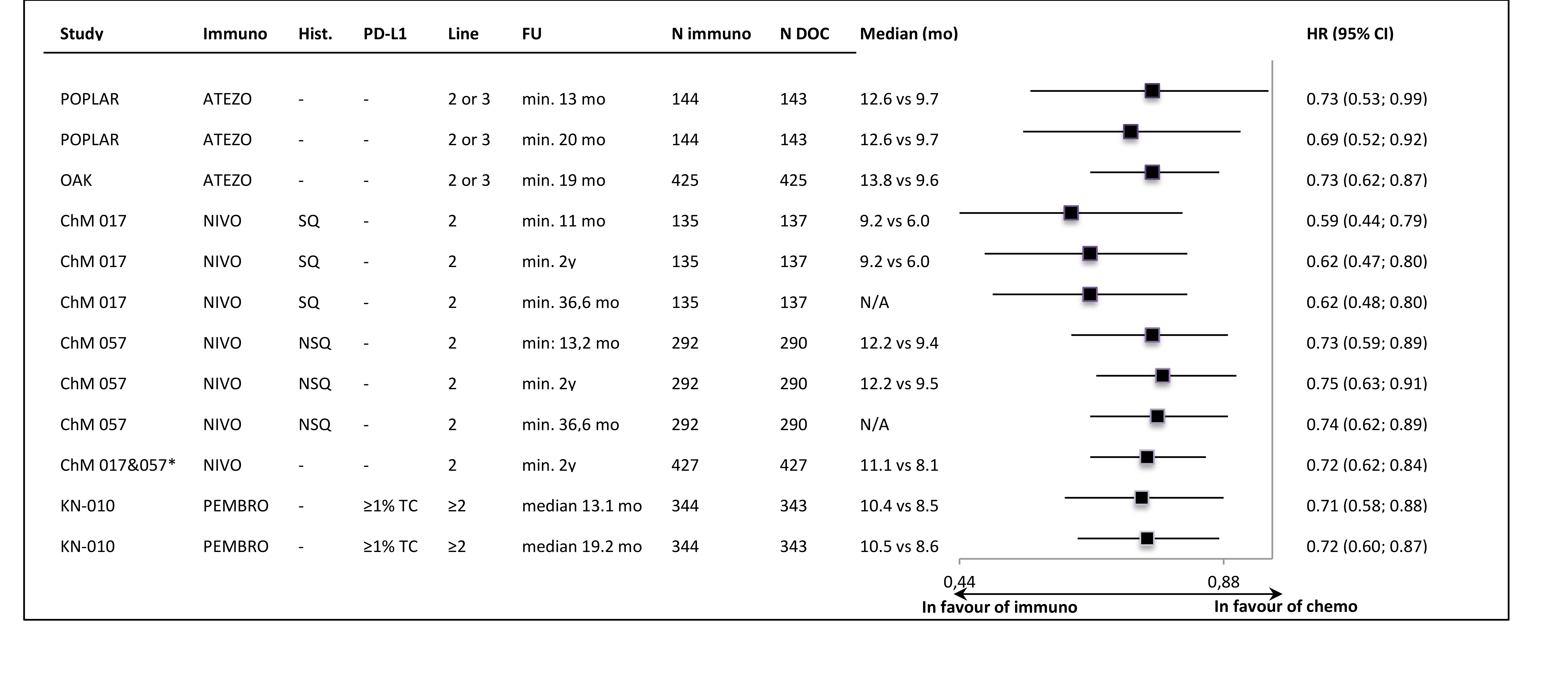
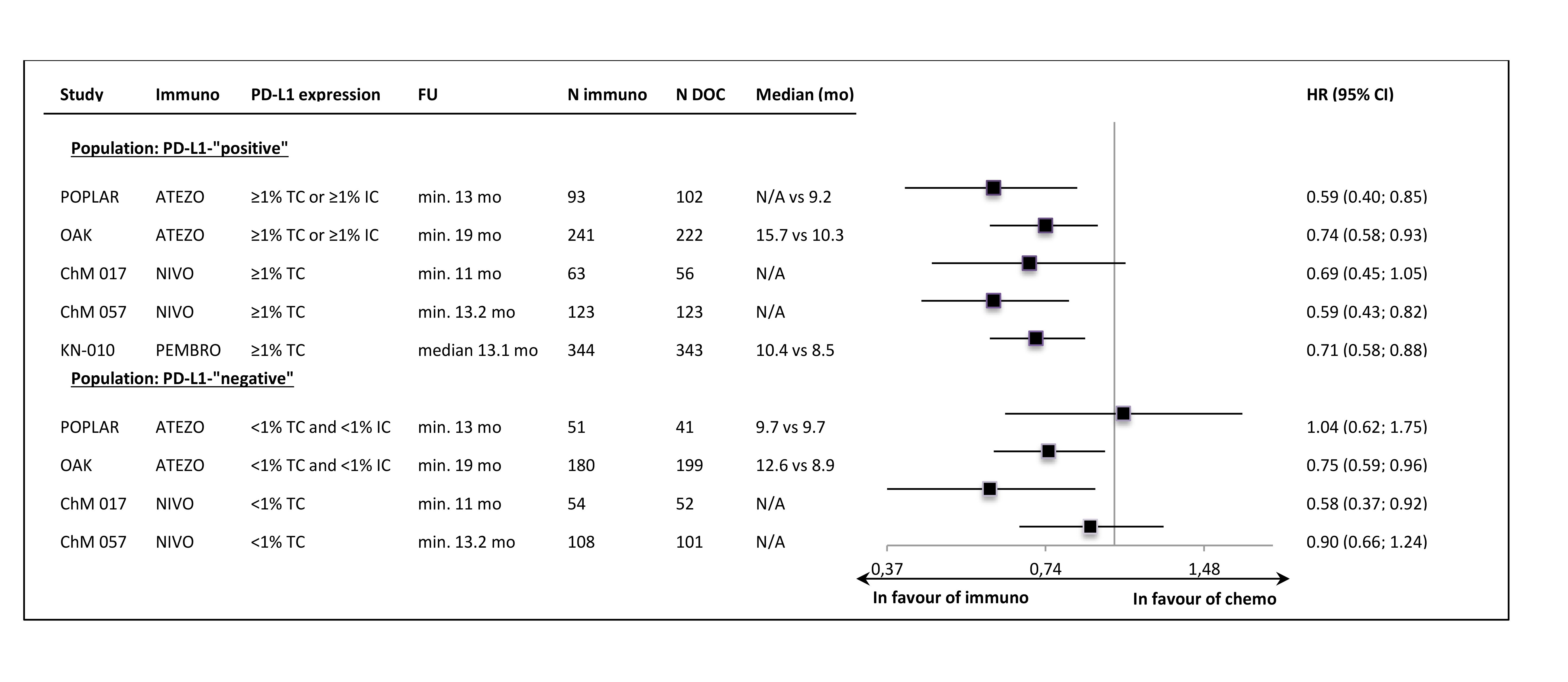
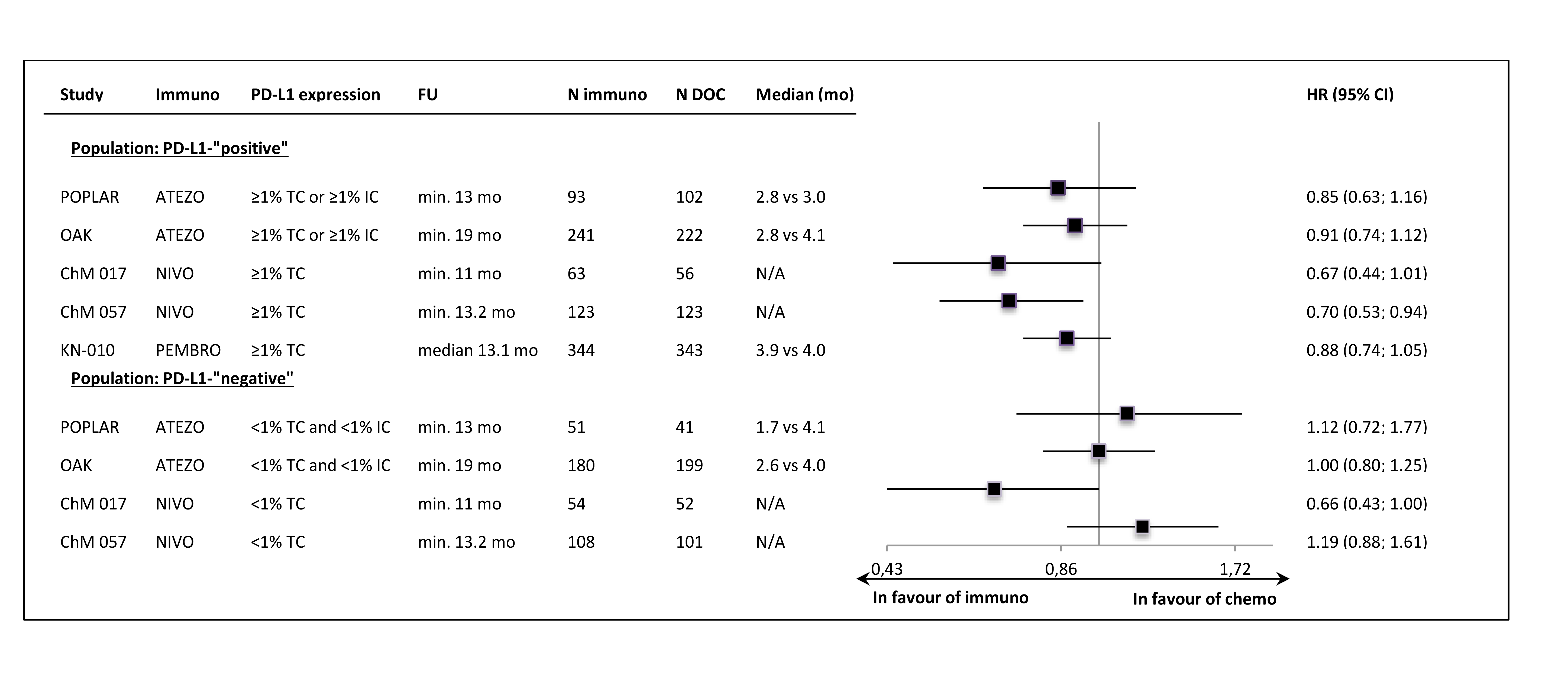
In all of the trials the primary ITT analyses showed significant OS improvement, with very similar HR values (around 0.72) in most of the studies. The lower HR (0.59) was noted in the sample of 2nd line, squamous carcinoma patients (CheckMate 0.17). The exclusion of PD-L1-“negative” patients from the Keynote-010 study did not apparently influence the size of the OS effect size, in comparison to the unselected samples. The updated OS results, available for most of the trials, showed that the effect of anti-PD-1/PD-L1 immunotherapy was maintained in a longer follow-up time (Figure 1.). The Figure 2. shows the results of the primary OS analyses in PD-L1-expression subgroups.
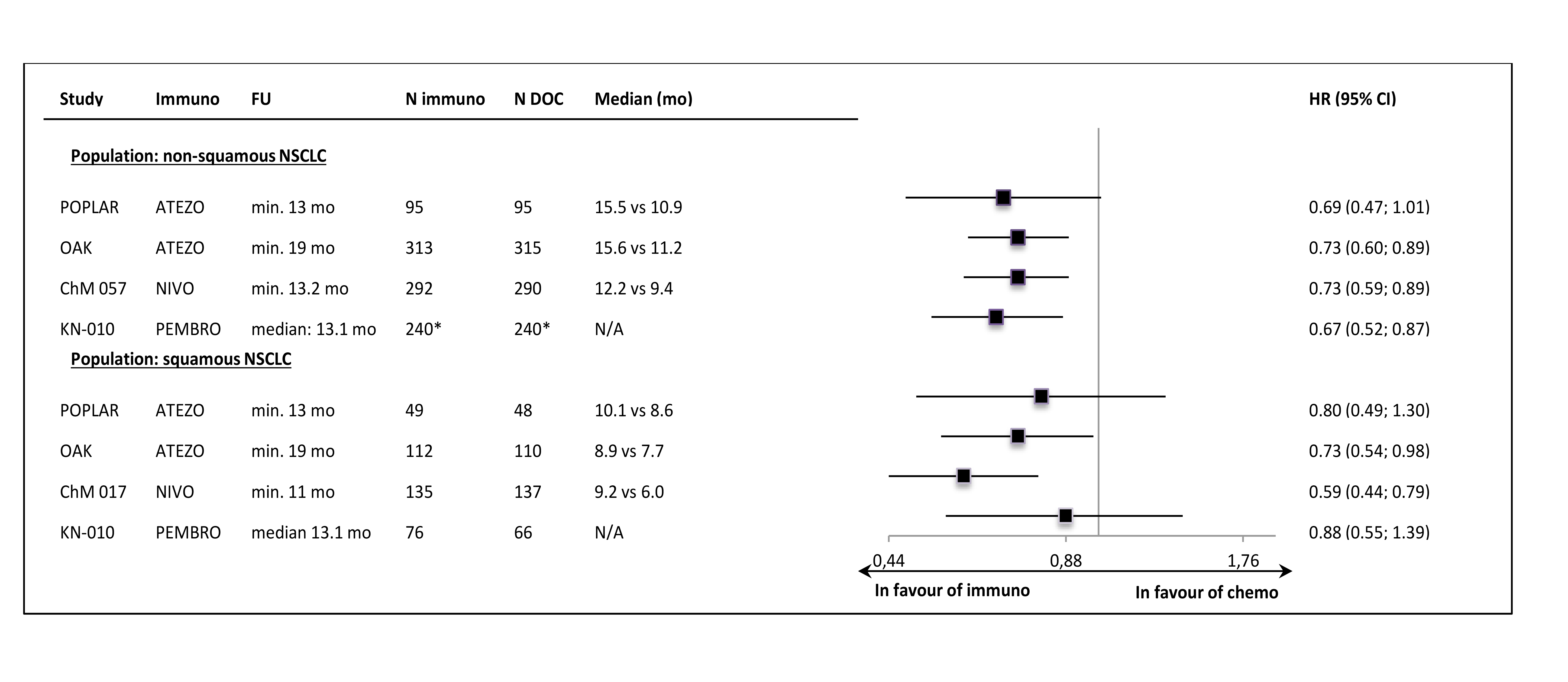
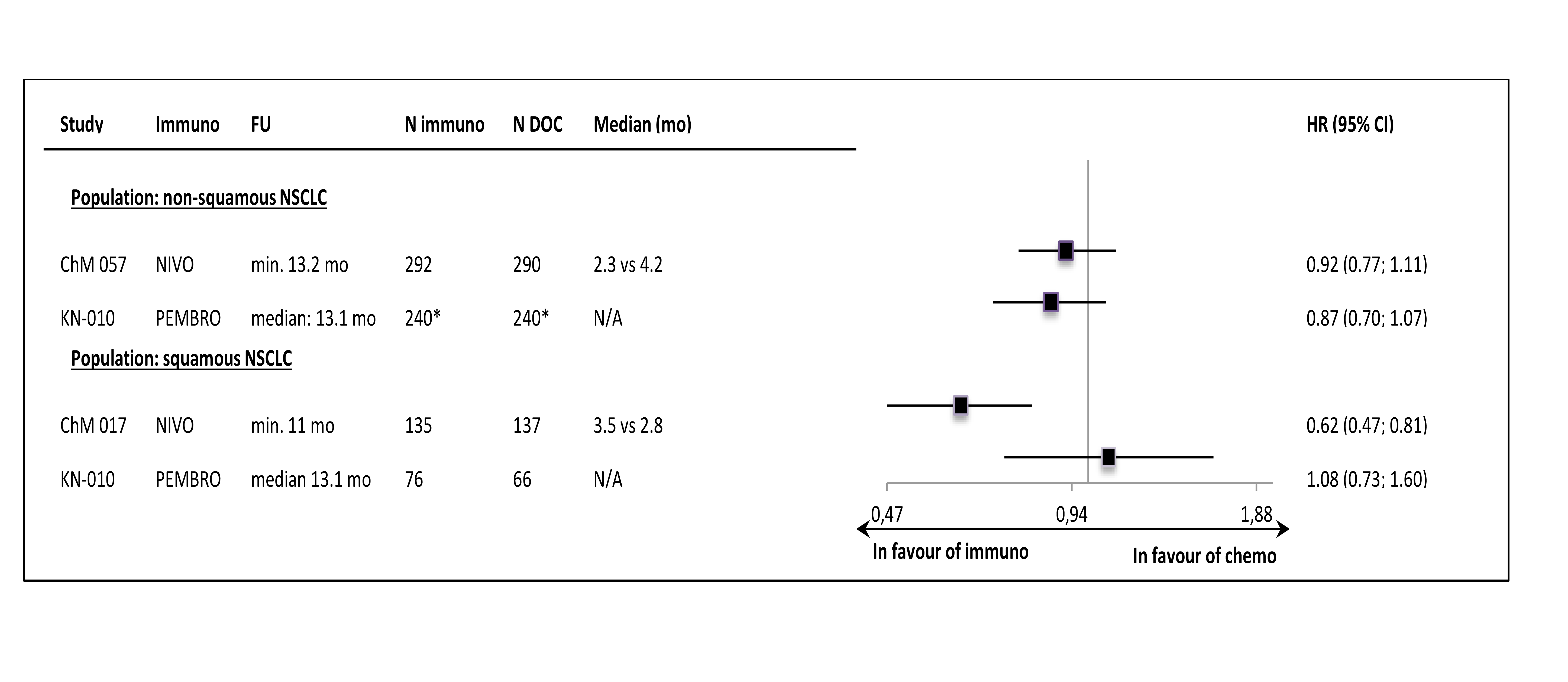
Despite the clinical heterogeneity of the samples and the variability in the definitions of PD-L1-“positivity” the results in PD-L1-“positive” patients, showing OS improvement in this subgroup, are consistent. The result did not reach the significance level in only one trial (CheckMate 017), though with the smallest sample size, the analysis could have been underpowered. In contrast, the results in PD-L1-“negative” subgroups were highly variable, with HR values in range from 0.58 to 1.04, with no obvious relation to the baseline between-study differences (though patients’ numbers are very small). The OS improvement was consistently shown in both histology subgroups, with a slightly higher variability of HR estimates in less numerous subgroups with squamous tumours (Figure 3.).
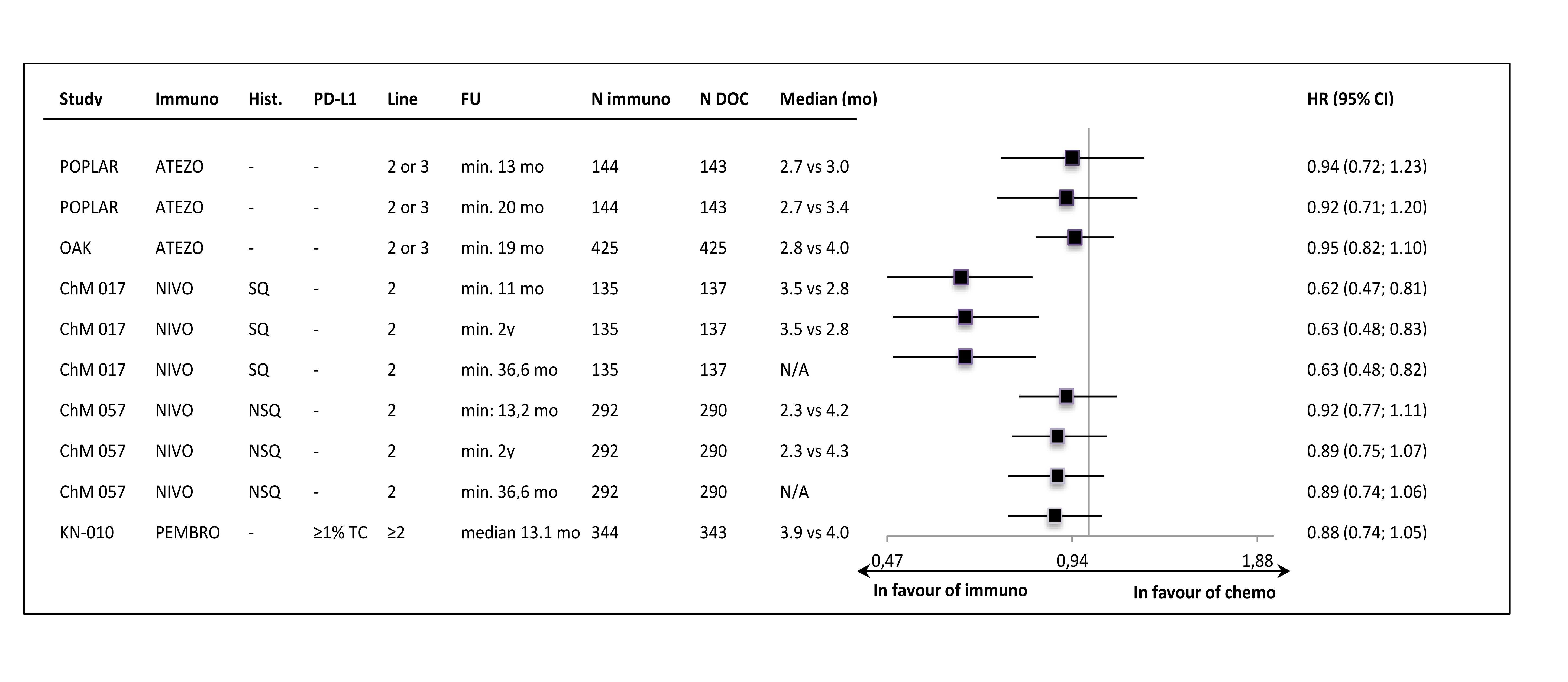
The PFS per RECIST v1.1 was not improved in most of the samples, with the HR values close to 0.90 and median PFS values lower in control groups (Figure 4.).
Figure 4. Progression-free survival per RECIST v1.1 in the intention-to-treat populations of the studies.
The only exception was the result in the CheckMate 017 trial (2nd line, squamous carcinoma patients only), where the significant PFS improvement was shown. However, in the second nivolumab study (CheckMate 057, non-squamous subtypes) the PFS result was very similar to those obtained in the atezolizumab and pembrolizumab trials. In PD-L1-“positive” subgroups the numerical, between-group differences in PFS were slightly more pronounced than in ITT populations, but most of the HRs did not reached statistical significance. In PD-L1-“negative” subgroup HR estimates were consistently around unity, with the exception of CheckMate 017 trial result (Figure 5.).
Figure 5. Progression-free survival per RECIST v1.1 in the PD-L1-“positive” and PD-L1-“negative” subgroups.
The PFS results in histology subgroups were available only for nivolumab and pembrolizumab studies; in the squamous subtype patients the effect was shown in the CheckMate 017 trial but it was not found in the Keynote-010 study (Figure 6.).
Progression-free survival, as assessed by modified criteria of tumour response
The median PFS values per RECIST v1.1 and per modified criteria of response, available from the POPLAR and the Keynote-010 studies are gathered in Table 3.
Table 3. Median progression-free survival determined according to RECIST v1.1 criteria and modified criteria of tumour response.
|
Study |
Immunotherapy |
Population/subgroup |
Follow-up |
N |
RECIST v1.1 |
Modified criteria* |
|
Median (95% CI) [mo] |
Median (95% CI) [mo] |
|||||
|
POPLAR [27] |
ATEZO |
ITT |
min. 13 mo |
144 |
2.7 (2.0, 4.1) |
4.3 (3.9, 7.0) |
|
POPLAR [27] |
ATEZO |
≥1% TC or ≥1% IC |
min. 13 mo |
93 |
2.8 (2.6, 5.5) |
6.8 (4.1, 8.5) |
|
POPLAR [27] |
ATEZO |
<1% TC and <1% IC |
min. 13 mo |
51 |
1.7 (1.4, 4.2) |
4.1 (1.6, 4.4) |
|
Keynote-010 [38] |
PEMBRO |
ITT (≥1% TC) |
median 13.1 mo |
344 |
3.9 (3.1, 4.1) |
4.9 (4.0, 5.9) |
* the immune-modified RECIST criteria (imRECIST) in the POPLAR study and the immune-related response criteria (irRC) in the Keynote-010 study
Abbreviations: ATEZO – atezolizumab; min. – minimum; mo – months; PEMBRO - pembrolizumab; PFS – progression-free survival; RECIST - Response Evaluation Criteria In Solid Tumours
In the POPLAR study, in which MAH-developed immune-modified RECIST criteria were used, median PFS estimates per imRECIST were consistently prolonged in comparison to the results of classical RECIST assessment. The difference was particularly pronounced in PD-L1-“positive” subgroup (4 months). In the Keynote-010 trial, using published irRC criteria, the difference was also observed, though smaller in size (1 month).
In the POPLAR trial the imRECIST criteria were applied only to the immunotherapy group. As a consequence, the HR for PFS per imRECIST versus chemotherapy group was not calculated. Unlike in the atezolizumab trial, in the Keynote-010 study the irRC was used in both groups. Resulting HR for PFS per irRC was 0.76 (95% CI: 0.64; 0.92), showing, in contrast to the primary PFS analysis [HR = 0.88 (0.74, 1.05)], the statistically significant PFS improvement in immunotherapy-treated patients [38]. Of note, the irRC assessment resulted in a slight shift of median PFS also in the chemotherapy group; from 4.0 (95% CI: 3.1, 4.2) months in the primary analysis per RECIST v1.1 to 4.4 (95% CI: 4.0, 5.5) months per irRC [38].
Discussion
Our review showed that the results of anti-PD-1/PD-L1 checkpoint inhibitors trials in previously treated patients with advanced NSCLC generally follows the expected pattern of the long-term efficacy results, in which the significant and consistent OS benefit is not accompanied with clear PFS prolongation, if the progression is assessed per classical RECIST v1.1 criteria. Restricting the analyses to the subgroup of patients with tumours (and/or tumour-infiltrating immune cells) expressing PD-L1 shifted the hazard ratios of PFS events towards the immunotherapy benefit but did not cause a fundamental change in the general picture of PFS results. The exception was the CheckMate 017 trial, which showed both OS and PFS gain in ITT population, while the second nivolumab study – CheckMate 057 – showed the same pattern as the atezolizumab and pembrolizumab trials. The main distinctive features the CheckMate 017 sample were the inclusion of only squamous tumours, while the other study samples consisted mostly of non-squamous subtypes. It was also the only sample that did not include any patients treated with more than one previous systemic therapy. Nevertheless, with numerous sources of heterogeneity the number of the trials is not sufficient to indicate those that determined the higher PFS gain. Moreover, the PFS results for histological subgroups were not available for atezolizumab trials and pembrolizumab has not been studied in a RCT enrolling PD-L1-“negative” patients and the first assessment of the tumour response in the atezolizumab trials was scheduled for earlier time point than in the remaining studies, what could have influenced the differences in the shapes of the PFS curves. All of those limitations precludes any strong inference with regard to the between-study differences in the results.
In spite of the heterogeneity of the patients’ samples and some methodological features, our review clearly showed that the use of the classical RECIST criteria in the assessment of the disease progression was not sufficient to fully capture the benefit of anti-PD-1/PD-L1 immunotherapy in advanced non-small-cell lung cancer patients. All of the anti-PD-1/PD-L1 agents prolonged overall survival, in the ITT populations, as well as across the histology and PD-L1-expression subgroups, while this unequivocal benefit was not invariably mirrored with the PFS gain. The existence of this pattern might not be obvious in a lung cancer immunotherapy, with the scarce data on pseudoprogression rates in this condition, possibly lower than in melanoma patients [13, 39]. In our sample of five RCTs we were able to identify the results of the PFS assessment with modified response criteria in only two studies. Moreover, the criteria used differed between those studies. The POPLAR study utilized the criteria developed specifically for the atezolizumab clinical programme [27], while the Keynote-010 used the irRC [9]. In addition, only the latter trial provided the result of the modified assessment for the chemotherapy group. The findings are in line with those expected on the basis of previous findings in melanoma immunotherapy: the median PFS estimates per modified criteria were prolonged in the immunotherapy arms as compared to the PFS per RECIST outcomes and the difference versus chemotherapy arm was pronounced (the HR estimate, available only from the Keynote-010 trial, reached the statistical significance). However, the RCT data on the difference between the assessment with the classical and modified criteria of tumour response in the second-line advanced NSCLC population are scant. The current use of the anti-PD-1/PD-L1 inhibitors in the routine practice allows for further, real-world research. The recent retrospective, single-centre study of 56 advanced NSCLC patients treated with nivolumab, that used RECIST1.1 and irRECIST1.1 criteria, found no cases of pseudoprogression during the treatment period of the study [13]. The response rate was identical between the two criteria, while the time-to-progression (TTP) by irRECIST1.1 was longer than TTP by RECIST1.1 [13]. The authors noted the limitations of their initial, short-term investigation and emphasized the need for further trials to address long-term effect of PD-1 inhibitor therapy on immune-related response characteristics in NSCLC [13].
Further research on the pattern of the immune response specific for NSCLC is important for the use of the immune checkpoint inhibitors in the clinical practice, as the premature diagnosis of a progressive disease may result in cases of treatment withdrawal in patients that might still benefit from the continuation of the immunotherapy. All of the RCTs included in our review allowed for the continuation of the immunotherapy, regardless of the occurrence of the disease progression according to the conventional RECIST v1.1 criteria, and the post-progression responses could have significantly contributed to the overall survival benefit. The exploratory analyses of the OAK trial data showed that treatment beyond progression with atezolizumab was associated with high frequency of stable or decreased target lesions and median post-progression OS of more than one year [40]. Moreover, the improved OS with anti-PD-L1 checkpoint inhibitor treatment was seen in the subgroups of patients with the best overall response of a stable or progressive disease [41, 42]. Thus it seems reasonable that the clinical decisions should take into account the possibility of the appearance of the non-classical pattern of response in an immunotherapy-treated patient with NSCLC. However, with the lack of the agreement with respect to the unified set of response criteria adequate for the lung cancer immunotherapy assessment and the European guidelines still recommending the response evaluation according to RECIST v1.1 approach [14], the risk of premature discontinuation of the immunotherapy treatment seems to be considerable, particularly in the countries where the conditions of use for the novel drug treatments are detailed and rigid, due to the budgetary restrictions. That in turn may lead to the reduction of the clinical benefit below the expected on the basis of the clinical trials and result in not cost-effective use of those innovative treatments.
Our review has several limitations. Firstly, the approach we adopted to the exploration of the sources of heterogeneity was purely descriptive. We did not use any statistical, meta-regression methods, which were not deemed adequate in such a small sample of RCTs. Shown differences between samples’ characteristics should be considered rather the inspiration for further research than a certain cause of the observed between-trial differences in the results. The description of the results available for the histology and PD-L1 expression subgroups, though incomplete, may provide some more data for this purpose. However, with respect to the PD-L1 expression level, we gathered OS and PFS results only for subgroups defined on the basis of the detection (or non-detection) of at least minimal PD-L1 expression (as defined by researchers in each trial), thus we did not explore the possible impact of further differences in the PD-L1 expression level (e.g. we did not extracted data for “high expression” subgroups). Secondly, we focused on the endpoints that form the key basis for the pre-approval assessment of new cancer therapies, namely OS and PFS. For this reason we did not analyse the other clinical results, like response rates or duration of response data, which are undeniably important for the complete description of the specific pattern of the tumour response to the immunotherapy. Finally, our conclusions are based on the assessment of the PD-1/PD-L1 immune checkpoint inhibitors in previously treated patients with advanced NSCLC and should not be directly applied to the other types of immunotherapy or clinical conditions.
Of note, there are numerous recently published analyses that pooled the immunotherapy results across different patients’ populations and immunotherapeutic agents, ignoring heterogeneity to draw the general conclusions on efficacy and/or safety of this type of treatment [43 – 53]. Although we do not dispute such approach, our review indicates that between-study differences in the eligibility criteria and in the other methodological aspects, may be considerable. Hence, any pooled estimates and indirect comparisons between immunotherapeutic agents should be treated with necessary caution.
Conclusions
The value of progression-free-survival per RECIST v1.1 as an endpoint may be limited in cancer immunotherapy evaluation, due to the complexity of the immune mechanisms, resulting in the specific pattern of tumour response to anti-PD-1/PD-L1 antibodies. Our review showed that the results of anti-PD-1/PD-L1 checkpoint inhibitors trials in previously treated patients with advanced NSCLC generally follows the expected pattern of the long-term efficacy results, in which the significant and consistent OS benefit is not accompanied with clear PFS prolongation, if the progression is assessed with the conventional criteria of tumour response. The use of the modified response criteria resulted in prolonged median PFS estimates in the immunotherapy-treated patients. In the routine practice the premature diagnosis of a progressive disease may result in treatment withdrawal in patients that might benefit from the continuation of the immunotherapy. Thus further studies on the pattern of the immune response specific for non-small-cell lung cancer patients that will facilitate the consensus on the most relevant modified criteria of tumour response in this clinical condition are urgently needed.
Acknowledgements
This study was supported by a grant from Roche Polska Sp. z o.o., Warsaw, Poland.
- Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388-97.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205-16.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-47.
- WHO handbook for reporting results of cancer treatment. Geneva (Switzerland): World Health Organization Offset Publication No. 48, 1979.
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207-14.
- Dranitsaris G, Cohen RB, Acton G, Keltner L, Price M, Amir E, et al. Statistical Considerations in Clinical Trial Design of Immunotherapeutic Cancer Agents. J Immunother. 2015;38(7):259-66.
- Johnson P, Greiner W, Al-Dakkak I, Wagner S. Which Metrics Are Appropriate to Describe the Value of New Cancer Therapies? Biomed Res Int. 2015;2015:865101.
- Miernik K, Czarny-Ozga I, Walczak J. Assessment of quality and clinical significance of endpoints in cancer immunotherapy. Journal of Health Policy and Outcomes Research 2015; 2:45-66.
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412-20.
- Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936-43.
- Nishino M, Gargano M, Suda M, Ramaiya NH, Hodi FS. Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer. 2014;2:17.
- Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment. Eur J Radiol. 2015;84:1259–68.
- Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Jänne PA, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84.
- Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v1-v27.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer Version 1.2018 - November 17, 2017. Available from: NCCN.org [Accessed November 20, 2017].
- Nishino M. Immune-related response evaluations during immune-checkpoint inhibitor therapy: establishing a “common language” for the new arena of cancer treatment. Journal for Immunotherapy of Cancer. 2016;4:30.
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565-70.
- Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res. 2015;21(5):976-84.
- He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer. Scientific Reports. 2015;5:13110.
- European Medicines Agency (EMA). Product information: Opdivo (nivolumab). Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003985/human_med_001876.jsp&mid=WC0b01ac058001d124 [Accessed November 20, 2017].
- European Medicines Agency (EMA). Product information: Tecentriq (atezolizumab). Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004143/human_med_002166.jsp&mid=WC0b01ac058001d124 [Accessed November 20, 2017].
- European Medicines Agency (EMA). Product information: Keytruda (pembrolizumab). Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003820/human_med_001886.jsp&mid=WC0b01ac058001d124 [Accessed November 20, 2017].
- Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(30):3484-3515.
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al.; POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-46.
- Smith DA, Vansteenkiste JF, Fehrenbacher L, Park K, Mazieres J, Rittmeyer A, et. al. Updated survival and biomarker analyses of a randomized phase II study of atezolizumab vs docetaxel in 2L/3L NSCLC (POPLAR). J Clin Oncol 34, 2016 (suppl; abstr 9028).
- Mazieres J, Fehrenbacher L, Rittmeyer A, Spira AI, Park K, Smith DA, et. al. Non-classical response measured by immune-modified RECIST and post-progression treatment effects of atezolizumab in 2L/3L NSCLC: results from the randomized phase II study POPLAR. J Clin Oncol 34, 2016 (suppl; abstr 9032).
- Mazieres J, Fehrenbacher L, Rittmeyer A, Spira AI, Park K, Smith DA, et. al. Non-classical response measured by immune-modified RECIST and post-progression treatment effects of atezolizumab in 2L/3L NSCLC: results from the randomized phase II study POPLAR. American Society of Clinical Oncology (ASCO) Meeting – June 3-7, 2016, Chicago, IL, USA. Poster #355.
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al.; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265.
- Gadgeel S, Kowanetz M, Zou W, Hirsch FR, Kerr KM, Gandara DR, et al. Clinical efficacy of atezolizumab (Atezo) in PD-L1 subgroups defined by SP142 and 22C3 IHC assays in 2L+ NSCLC: Results from the randomized OAK study. Ann Oncol. 2017;28 (suppl. 5): v460–v496, abstr 1296O.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-35.
- Barlesi F, Steins M, Horn L, Ready N, Felip E, Borghaei H, et al. Long-term outcomes with nivolumab (Nivo) vs docetaxel (Doc) in patients (Pts) with advanced (Adv) NSCLC: CheckMate 017 and CheckMate 057 2-y update. Annals of Oncology 2016;27(suppl. 6): vi422, abstr 1215PD.
- Felip Font E, Gettinger SN, Burgio MA, Antonia SJ, Holgado E, Spigel DR, et al. Three-year follow-up from CheckMate 017/057: Nivolumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer (NSCLC). Annals of Oncology 2017; 28(suppl. 5): v462, abstr 1301PD.
- Felip Font E, Gettinger SN, Burgio MA, Antonia SJ, Holgado E, Spigel DR, et al. Three-year follow-up from CheckMate 017/057: Nivolumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer (NSCLC). Presented at the European Society for Medical Oncology 42nd Congress; September 8–12, 2017; Madrid, Spain. Poster 1301PD.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627-39.
- Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017:JCO2017743062.
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-50.
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab (pembro) vs docetaxel (doce) for previously treated, PD-L1-expressing NSCLC: Updated outcomes of KEYNOTE-010. Annals of Oncology 27 (suppl 6): vi552-vi587, 2016, abstr LBA48.
- Pembrolizumab for treating advanced or recurrent PD-L1 positive non-small-cell lung cancer after progression with platinum-based chemotherapy [ID840]. Merck Sharp & Dohme: Evidence submission. 24th March 2016. In: The National Institute for Health and Care Excellence (NICE). Single Technology Appraisal. Pembrolizumab for treating PD-L1-positive non-small-cell lung cancer after platinum-based chemotherapy [ID840]. Committee Papers. Published on 4th October 2016. Available from: https://www.nice.org.uk/guidance/ta428/evidence [Accessed November 13, 2017].
- Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015 Nov 1;33(31):3541-3.
- Gandara DR, von Pawel J, Sullivan RN, Helland A, Han J-Y, Ponce Aix S, et. al. Impact of atezolizumab (atezo) treatment beyond disease progression (TBP) in advanced NSCLC: Results from the randomized phase III OAK study. J Clin Oncol 35, 2017 (suppl; abstr 9001).
- de Marinis F, Barlesi F, Rittmeyer A, Han J-Y, Kozloff M, Spira A, et. al. Survival and safety of atezolizumab by best overall response (BOR) in the phase III NSCLC OAK study. Abstract Book of the 42nd ESMO Congress (ESMO 2017) 8–12 September 2017, Madrid, Spain. Annals of Oncology 2017;(28):suppl 5,. abstr 1310P.
- de Marinis F, Barlesi F, Rittmeyer A, Han J-Y, Kozloff M, Spira A, et. al. Survival and safety of atezolizumab by best overall response (BOR) in the phase III NSCLC OAK study. European Society for Medical Oncology 2017 (ESMO 2017); September 8-12; Madrid, Spain. Poster #1310P.
- Abdel-Rahman O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: A meta-analysis. Crit Rev Oncol Hematol 2016; 101:75-85.
- Aguiar PN, Santoro IL, Tadokoro H, De Lima Lopes G, Filardi BA, et al. The role of PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: A network meta-analysis. Immunother 2016; 8(4):479-488.
- Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017; 12(2):403-407.
- Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist 2017; 22(4):470-479.
- Sheng Z, Zhu X, Sun Y, Zhang Y. The efficacy of anti-PD-1/PD-L1 therapy and its comparison with EGFR-TKIs for advanced non-small-cell lung cancer. Oncotarget. 2017 Jun 8;8(34):57826-57835.
- Wang C, Yu X, Wang W. A meta-analysis of efficacy and safety of antibodies targeting PD-1/PD-L1 in treatment of advanced nonsmall cell lung cancer. Medicine (Baltimore) 2016; 95(52):e5539.
- Yang Y, Pang Z, Ding N, Dong W, Ma W, Li Y, et al. The efficacy and potential predictive factors of PD-1/PD-L1 blockades in epithelial carcinoma patients: A systematic review and meta analysis. Oncotarget 2016; 7(45):74350-74361.
- Yu D-P, Cheng X, Liu Z-D, Xu S-F. Comparative beneficiary effects of immunotherapy against chemotherapy in patients with advanced NSCLC: Meta-analysis and systematic review. Oncol Lett 2017; 14(2):1568-1580.
- Zhang T, Xie J, Arai S, Wang L, Shi X, Shi N, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 2016; 7(45):73068-73079.
- Zhou G-W, Xiong Y, Chen S, Xia F, Li Q, Hu J. Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer A meta-analysis of randomized clinical trials. Medicine 2016; 95(35).
- Zhu L, Jing S, Wang B, Wu K, Shenglin MA, Zhang S. Anti-PD-1/PD-L1 Therapy as a Promising Option for Non-Small Cell Lung Cancer: a Single arm Meta-Analysis. Pathol Oncol Res 2016; 22(2):331-339.