VaxigripTetra (Quadrivalent Influenza Vaccine) – evidence for clinical and economic benefits from vaccination in population aged 65 and over in Poland
-
Copyright
© 2018 PRO MEDICINA Foundation, Published by PRO MEDICINA Foundation
User License
The journal provides published content under the terms of the Creative Commons 4.0 Attribution-International Non-Commercial Use (CC BY-NC 4.0) license.
Authors
Background: Vaccination is considered to be the most effective way of preventing influenza related-illnesses and their complications, which is particularly important in elderly population (aged 65+), often characterized with more rapid and severe clinical course of infection. Quadrivalent influenza vaccines (QIV) have been developed to avoid the poor antigenic match observed in the trivalent formulation vaccine (TIV).
Methods: The study assess the immunogenicity, cost-effectiveness and impact on the Polish budget of inactivated quadrivalent influenza vaccine (VaxigripTetra), assuming its reimbursement in the active immunization of adults 65 years of age and older.
Results: As clinical results have shown, non-inferior immunogenicity of QIV to each TIV for the shared strains and superior immunogenicity of QIV to each TIV for the alternate B strain. The inactivated QIV was well tolerated. VaxigripTetra is a highly cost-effective option compared to the strategy of “no vaccination” (ICUR=14,988 PLN/QALY – payer’s + patients’ perspective [PPP+P], ICUR=6,838 PLN/QALY – payers’ perspective [PPP]) and also to TIV (ICUR=121,418 PLN or ICUR=114,360 PLN, respectively). The increase of the public payer’s expenditures – 9.0-23.3 million PLN annually – will result in additional health effects: 1,091 – 4,805 GP visits, 175-771 hospitalizations and 74-326 deaths avoided per year.
Conclusions: VaxigripTetra reimbursement could trigger a faster switch from TIVs to QIVs and at the same time could increase the vaccine coverage of the elderly population in Poland, which would lessen the ILI-related negative impact on that population at a reasonable cost to the healthcare system, while providing some savings to society.
Background
Vaccination is considered to be the most effective option for preventing influenza related-illnesses (ILI) and their complications. The consequences of an infection can be severe, especially bacterial infections impairing functioning of important organs. This often leads to hospitalization and even the patient’s death. The number of hospitalizations due to influenza in season 2015/2016 in Poland was approximately 16,000, which was much more than the total number of the remaining most common causes of hospitalization due to infectious diseases (chickenpox, hepatitis B and viral meningitis), which amounted to approximately 3,000 [1, 2].
Seasonal influenza epidemics can seriously affect all populations, but the highest risk of complications is observed among pregnant women, children aged 6-59 months, the elderly or individuals with specific chronic medical conditions such as HIV/AIDS [3]. The recommendation of The College of Family Physicians in Poland emphathises that one of the medical reasons for vaccination against influenza is age over 50 years or chronic diseases, which are very common in older people, while an epidemiological reason is a stay in nursing homes and health resorts [1]. Recommendations of foreign panels of experts are similar [4, 5].
According to European Centre for Disease Prevention and Control (ECDC) data, all 30 countries in the European Union and the European Economic Area recommended seasonal influenza vaccination for the older age groups in 2014-15, but the specified age differed between countries. Eighteen of them, among others Great Britain, France, Spain, Italy, Norway, Sweden, Romania, Bulgaria and the Czech Republic, recommended vaccination for individuals ≥ 65 years of age [6].
For many years, the commonly used vaccine in influenza immunization programs was trivalent formulation (TIV), which included 2 influenza A strains virus (A/H1N1 and A/H3N2) and one B virus strain. However, because of worldwide circulation of two distinct lineages of influenza B (the Victoria and Yamagata lineages) in the same influenza season since 2000, predictions as to which B lineage would be dominant in the following season have been inadequate. From 2001 to 2011 these estimates were correctly forecasted in only 5 of the 10 seasons [7 – 9].
To address the limited cross-protection effect between those 2 antigen types and avoid poor antigenic match, the World Health Organization since 2012 has recommended a fourth strain to be included in seasonal influenza vaccines. In line with this recommendation, it is expected that quadrivalent vaccine (QIV), which contains 2 influenza A strains (A/H1N1 and A/H3N2) and 2 influenza B lineages (B/Victoria and B/Yamagata), could prove to be more effective in the control of seasonal influenza than trivalent vaccine [10 – 12].
Clinical data
Analysis of the clinical efficacy of influenza vaccines should be based on the assessment of immune responses elicited by the vaccine. It is recommended that evaluation of vaccine immunogenicity be carried out for each viral strain included in the vaccine. Immunogenicity in the case of anti-HA antibodies includes assessment of rates of seroconversion (SCR), rates of seroprotection (SPR) and geometric mean titre ratio (GMTR) [13, 14].
The immunogenicity of quadrivalent vaccines was assessed by conducting a systematic review and quantitative synthesis of its results. We searched MEDLINE, EmBase and The Cochrane Central Register of Controlled Trials, using terms defining quadrivalent influenza vaccines and the target population of older people (last updated 23 August 2017).
The analysis covered the population of adults 65 years of age and older, but randomized controlled trials evaluating adults 60 years of age and older (or presenting results in that subgroup) were also included, because of differences in definition of elderly people. The analysis compared the immunogenicity and safety of quadrivalent vs trivalent influenza vaccines.
Two of the included studies evaluated the VaxigripTetra product – in the Pepin 2013 trial [15] the initial formulation was used and in the GQM11 study [16] the final formulation. Both studies were randomized and double-blinded.
In the Pepin 2013 trial, in the case of all four vaccine strains, antibody responses to the QIV were non-inferior to the response to the TIV for the shared strains, in terms of GMT ratio and SCR between QIV vs TIV groups. For both B strains, post-vaccination antibody responses to the QIV were superior to the responses to the TIVs lacking the corresponding B strain. Similarly, for all QIV lots analysed in GQM11 study, haemagglutination-inhibition geometric mean titres were non-inferior to those for the pooled TIVs for the three shared strains and superior for the additional B strain when absent from the comparator TIV.
In the Pepin 2013 trial, solicited reactions, unsolicited adverse events and serious adverse events were similar for the QIV and pooled TIV groups. No treatment-related serious adverse events were reported. Two deaths that were observed in QIV group were unrelated to the vaccine.
Our analysis also assessed meeting the European Medicines Agency (EMA) and Food and Drug Administration (FDA) vaccine effectiveness criteria by the identified studies. As Table 1 shows, in the two major studies – Pepin 2013 and GQM11 – the results for all analysed endpoints (SPR, SCR and GMTR) meet these criteria.
Table 1. Meeting EMA and FDA vaccine effectiveness in the main studies including in clinical analysis.
A1 – A/H1N1; A2 – A/H3N2; B1 – B/Victoria; B2 – B/Yamagata; SPR – seroprotection; SCR – seroconversion; GMTR – geometric mean titre ratio; FDA – Food and Drug Administration; EMA – European Medicines Agency; CI – confidence interval
Cost-effectiveness assessment
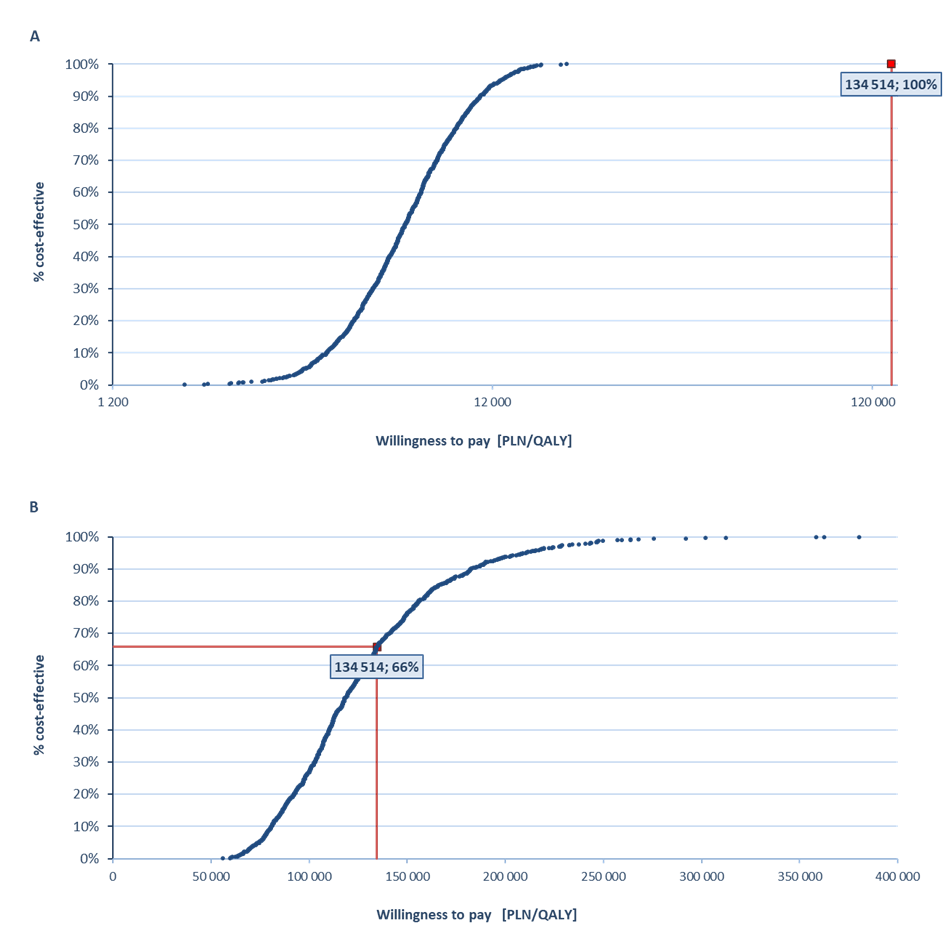
One of the key criteria taken into account during the process leading to reimbursement decisions in Poland is the monetary value of the health benefit of the assessed medical technology (incremental cost-utility ratio, ICUR). A health technology is considered to be cost-effective if the cost of generating an additional quality-adjusted life year does not exceed three times the Gross Domestic Product (GDP) per capita (134 514 PLN/quality-adjusted life year [QALY], condition as for November 2017) [17].
Assessing the cost-effectiveness of quadrivalent vaccines was based on a published model developed by Creative-Ceutical for Canadian settings [18]. It was adapted by incorporating, where possible, Poland-specific costs and epidemiological and health outcomes data, attributable to influenza over a one year horizon, which reflected the “average” epidemic season of influenza. For example, the number of influenza-related visits were estimated based on data published by the National Institute of Public Health – National Institute of Hygiene (NIPH-NIH), while data on influenza-related deaths came from the EuroMOMO project (FluMOMO model) [19], which was in line with the recommendation of National Consultant on Epidemiology in Poland, considering NIPH-NIH’s data in this area as underreported.
As the most relevant comparator, the strategy of not vaccinating against influenza (“no vaccination”) was selected. Because trivalent influenza vaccines are available, but not reimbursed in Poland, they were used as an additional comparator in the economic analysis. Cost-utility analysis (CUA) was chosen as the most appropriate analytical technique in the economic assessment of VaxigripTetra compared with both “no vaccination” and TIV.
The results of the base-case analysis were presented for the entire target population (6.7 million people over the age of 65, forecast for 2018). We assumed that vaccination coverage in the target population (both for QIV and TIV) would be on an average level calculated from several recent years in Poland (11.4% based on ECDC) [6].
The analysis was conducted from the public payer’s perspective (PPP), as well as the common perspective of public payer and patient (PPP+P), taking into account the direct medical costs of the health problem considered. The ex-factory (catalogue) price of VaxigripTetra (50.00 PLN per 1 vaccine) was obtained from the manufacturer.
Assuming the common perspective of PPP+P, the cost of an additional quality-adjusted life-year for the assessed intervention (QIV) is 14,988 PLN/QALY and is well below the cost-effectiveness threshold in Poland. In turn, from the perspective of PPP, the cost of obtaining an additional quality adjusted year of life for QIV is 6,838 PLN/QALY and is also below the Polish cost-effectiveness threshold (Table 2.).
Table 2. Results of cost-effectiveness analysis (per 1 vaccinated person); QIV vs “no vaccination”, PPP+P and PPP perspective.
|
Strategy |
Cost [PLN] |
QALY lost |
Incremental cost |
Incremental effect |
ICUR [PLN/QALY] |
|
PPP+P |
|||||
|
QIV |
152.93 |
0.0657 |
61.49 |
0.0041 |
14,988 |
|
„no vaccination” |
91.44 |
0.0698 |
|||
|
PPP |
|||||
|
QIV |
119.49 |
0.0657 |
28.05 |
0.0041 |
6,838 |
|
„no vaccination” |
91.44 |
0.0698 |
|||
When compared with trivalent influenza vaccines in both perspectives of PPP+P and PPP, the quadrivalent vaccine is cost-effective. The cost of obtaining an additional QALY in the population of adults 65 years of age and older is 121,418 PLN/QALY and 114,360 PLN/QALY, respectively (Table 3.).
Table 3. Results of cost-effectiveness analysis (per 1 vaccinated person); QIV vs TIV, PPP+P and PPP perspective.
|
Strategy |
Cost [PLN] |
QALY lost |
Incremental cost |
Incremental effect |
ICUR [PLN/QALY] |
|
PPP+P |
|||||
|
QIV |
152.93 |
0.0657 |
35.09 |
0.0003 |
121,418 |
|
TIV |
117.84 |
0.0660 |
|||
|
PPP |
|||||
|
QIV |
119.49 |
0.0657 |
33.05 |
0.0003 |
114,360 |
|
TIV |
86.44 |
0,0660 |
|||
QIV therapy remained cost-effective vs the “no vaccination” strategy in all sensitivity analysis scenarios from both perspectives (PPP/PPP+P). The results were most sensitive to the assumptions regarding hospitalization rates and deaths due to influenza in the elderly population and the effectiveness of vaccines against Influenza A and type B viruses.
The QIV strategy remained cost-effective compared with TIV in most sensitivity analysis scenarios, from both adopted perspectives (PPP/PPP+P). With the most conservative assumptions regarding vaccine effectiveness in the elderly population, the cost of additional QALY increased to 230,000 PLN (PPP) and 240,000 PLN (PPP+P).
In the probabilistic sensitivity analysis, the VaxigripTetra vaccine was cost-effective vs non-influenza strategy with a 100% probability (PPP+P/PPP) and cost-effective vs TIV with a probability of 57% (PPP+P) and 66% (PPP) (Figure 1).
Budget impact analysis (BIA)
The clinical course of influenza in elderly patients is often more rapid and severe, which has a direct impact on increase of health care expenditures in this group and the socio-economic burden [20, 21].
The most important social and economic consequences are: costs of outpatient treatment, hospitalization and medicines, reduced quality of life, absence at work of patient’s family members and overall significant productivity losses to society. It was estimated that in Poland during the non-epidemic season, the general direct costs related to influenza amount to over 40 million PLN and indirect costs to over 800 million PLN [1, 22].
The budget impact analysis was performed to estimate the future expenditures of the public payer (National Health Fund, NHF) in the event that VaxigripTetra (inactivated quadrivalent influenza vaccine) would be publicly funded in Poland for the prevention of seasonal influenza in population over 65 years of age.
The incremental budget impact of VaxigripTetra reimbursement was estimated by comparing two alternative scenarios: “current” – vaccine is not reimbursed in Poland and “new” – VaxigripTetra reimbursed in Poland with the 50% level of patient co-payment. The analysis was performed from a public payer perspective and the public payer and patient perspective over a 4-year time horizon (epidemic seasons: 2018/2019, 2019/2020, 2020/2021 and 2021/2022).
In the base case analysis, only quadrivalent influenza vaccines – VaxigripTetra and the competing product Influvac Tetra® were considered. In BIA, QIV unit costs were differentiated by the category of vaccine availability (pharmacy sales, influenza prevention programs implemented by local government, purchase of vaccines at clinical centres). The analysis was performed in three variants: base-case (most probable), low and high.
In Poland, influenza vaccines are currently most often purchased in health centres and only 32% of vaccines administered in the elderly population comes from pharmacy sales. However, it's expected that reimbursement of VaxigripTetra would result – as a consequence of reduced pharmacy price – in growth in the pharmacy share, without affecting the availability of the vaccine from other providers (prevention programs implemented by local government, health centres). The estimated number of people 65 years of age and older vaccinated with VaxigripTetra under the new scenario is – taking into account all categories of vaccine availability – 605,968 (season 2018/2019), 775,464 (season 2019/2020), 956,423 (season 2020/2021) and 1,145,744 (season 2021/2022). Taking into account only pharmacy sales within the list of reimbursed drugs, the estimated numbers of people vaccinated with VaxigripTetra are 292,570, 451,793, 621,792 and 800,686, respectively, i.e. growth in pharmacy shares of 59%, 118%, 177% and 235% compared to the current scenario.
Public payer perspective
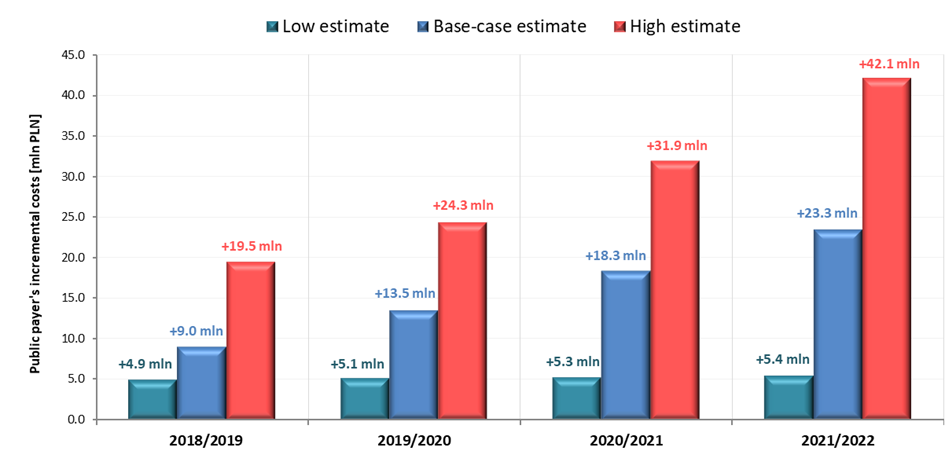
In the event of a positive decision regarding reimbursement of VaxigripTetra, the incremental costs to the public payer resulting from reimbursement of VaxigripTetra in 2018/2019 season, 2019/2020 season, 2020/2021 season and 2021/2022 season are 9.0 million PLN, 13.5 million PLN, 18.3 million PLN and 23.3 million PLN respectively. Detailed results of base, low and high-case variants are presented in Table 4 and in Figure 2.
Table 4. Results of budget-impact analysis, PPP perspective.
|
BIA scenario |
Cost (PPP) in scenarios: |
Season 2018/2019 |
Season 2019/2020 |
Season 2020/2021 |
Season 2021/2022 |
|
Base-case |
Incremental budget impact [PLN] |
9,001,775 |
13,494,711 |
18,291,699 |
23,336,902 |
|
Reimbursement of Vaxigrip Tetra [PLN] |
9,780,608 |
15,103,435 |
20,786,498 |
26,766,947 |
|
|
Low |
Incremental budget impact [PLN] |
4,937,135 |
5,098,966 |
5,271,630 |
5,435,888 |
|
Reimbursement of Vaxigrip Tetra [PLN] |
4,937,135 |
5,098,966 |
5,271,630 |
5,435,888 |
|
|
High |
Incremental budget impact [PLN] |
19,478,671 |
24,315,023 |
31,914,103 |
42,066,053 |
|
Reimbursement of Vaxigrip Tetra [PLN] |
20,257,504 |
25,923,747 |
37,019,115 |
49,084,825 |
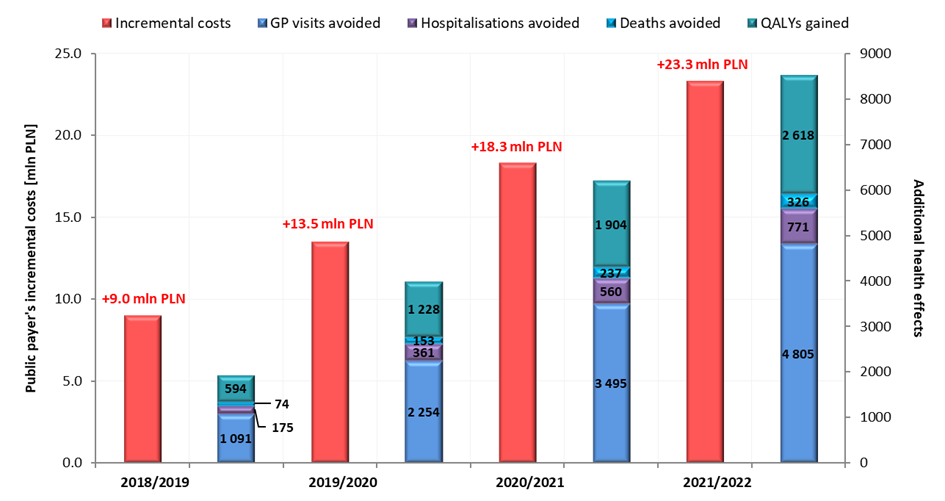
Notwithstanding, reimbursement of VaxigripTetra and increase of vaccine coverage in the target population may lead to the avoidance of 1,091 - 4,805 GP visits, 175 – 771 hospitalizations and 74 – 326 deaths per year in the population over 65 years of age (Figure 3.), which will generate savings of 0.8 – 3.4 million PLN annually.
Patients’ perspective
In case of a positive decision about reimbursement of VaxigripTetra, the incremental costs of patients in new scenario will decrease by 0.2 million PLN in 2018/2019 season and in following seasons will increase by 4.9 million PLN (2019/2020 season), 10.2 million PLN (2020/2021 season) and 19.8 million (2021/2022 season).
Summary
Despite the data presented above and the opinion of Advisory Committee of HTA [Health Technology Assessment] Agency in Poland, issued in 2009 [23], which recommends reimbursement of vaccination against seasonal influenza in high-risk groups, including people with chronic diseases, influenza vaccines have not been financed by the NHF in Poland so far. Patients can only be vaccinated against influenza free of charge in vaccination programs financed by local government units or private health insurance at workplaces.
It should be emphasized that of the 163 regional health policy programs assessed by The Agency for Health Technology Assessment and Tariff System up to 25 August 2017, 53.4% concerned assessment of the implementation of influenza vaccination in adults 65 years of age and older and 89% of the mentioned projects were assessed positively (sometimes after fulfilling additional conditions).
What is important, in many countries with GDP similar to Poland (Croatia, Estonia, Lithuania, Latvia and Portugal), influenza vaccination is reimbursed by public payer. In four of them, the refund with a different level of patient co-payment (0% or 50%) covers exactly the population analysed in our study, i.e. patients aged 65 and more.
Moreover, difficulty of access to fully free vaccination may be the reason that in Poland the Council of the European Union and the World Health Organisation’s level (75%) of vaccine coverage in population of people aged 65+ has still not been achieved. In the 2014/2015 season, mean vaccination coverage in this population was 13.4%, which is very low compared, for example, with Portugal or Hungary, where this percentage was approximately 2 and 4 times greater [6].
This situation could be changed by reimbursement of the VaxigripTetra product. The specificity of the health care system in Poland, in case of vaccines, permits applying for reimbursement via pharmacy with vaccine co-payment of 50%; however, even with this level of participation it is expected that vaccine availability will improve and vaccination coverage will widen. And even greater availability of the vaccine could be achieved via its funding through the list of medicines accessible free of charge for people aged 75 and over, which has been in operation in Poland since 2017.
As the literature data show, influenza vaccines are substantial for the prevention of influenza and influenza-like illness in elderly people, and VaxigripTetra could further extend that protection, by minimalizing the effect of seasonal mismatch to B virus strains, which has been proven in our analysis.
Moreover, we confirmed that replacing trivalent influenza vaccine or no vaccination strategies with VaxigripTetra in individuals aged 65 years or older results in additional health effects, such as avoided physician visits, hospitalization and influenza-related deaths, as well as additional years of quality-adjusted life. It should be noted here that our results are consistent with other economic analyses carried out in the neighbouring countries of the European Union, which indicate that QIV vaccine is cost-effective in the elderly population [24, 25].
Conclusions
As clinical results have shown, antibody responses to the inactivated QIV were superior to the responses to TIV for the unmatched strains and non-inferior for the matched strains. The QIV was also well tolerated without any safety concerns. The use of VaxigripTetra for seasonal influenza vaccination is a highly cost-effective option against the strategy of “no vaccination” and a cost-effective option against TIV in the population of adults 65 years of age and older.
This proves that by preventing infection from both influenza B lineages, QIV could help avoid more influenza cases, complications and deaths than TIV. Thus, replacing optional strategies by VaxigripTetra will actually be related to an increase in expenditure, but will first of all improve the health of the elderly population in Poland at a reasonable cost to the healthcare system, while providing savings to society.
Acknowledgements
This study was supported by a grant from Sanofi Pasteur Sp. z o.o., Warsaw, Poland. Dr. J. Lis, Mrs E. Bernaszuk and Mrs K. Wepsięć are employees of Sanofi- Aventis Sp. z o.o.
1. Makowiec-Dyrda M., Tomasik T. et al.: Profilaktyka i leczenie grypy. Wytyczne Kolegium Lekarzy Rodzinnych w Polsce (2016). Available from: http://www.klrwp.pl/strona/226/profilaktyka-i-leczenie-grypy-2016/pl.
2. Kuchar E. Prezentacja “Grypa 2017”. Available from: http://www.infozdrowie.org/images/pdf/2017/13_06_2017/1_kuchar.pdf.
3. Bekkat-Berkani R., Ray R., Jain V.K., Chandrasekaran V., Innis B.L.: Evidence update: GlaxoSmithKline's inactivated quadrivalent influenza vaccines. Expert Rev Vaccines. 2016; 15: 201-14.
4. Grohskopf L.A., Sokolow L.Z., Broder K.R. et al.: Prevention and Control of Seasonal Influenza with Vaccines Recommendations of the Advisory Committee on Immunization Practices - United States, 2016-17 Influenza Season. MMWR Recomm Rep 2016; 65: 1-52.
5. Harper S.A., Bradley J.S., Englund J.A. et al.: Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children--diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48: 1003-32.
6. European Centre for Disease Prevention and Control. Seasonal influenza vaccination in Europe. Vaccination recommendations and coverage rates in the EU Member States for eight influenza seasons: 2007–2008 to 2014–2015. Stockholm: ECDC; 2017. Available from: https://ecdc.europa.eu/sites/portal/files/documents/influenza-vaccination-2007%E2%80%932008-to-2014%E2%80%932015.pdf.
7. Ambrose C.S., Levin M.J.: The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012; 8: 81-88.
8. US Centers for Disease Control and Prevention. Seasonal influenza activity surveillance reports: 1999-2000 to 2010-2011 seasons. Available from: http://www.cdc.gov/flu/weekly/pastreports.htm.
9. Toback S.L., Rothstein E., Bhatt P., Carr W., Ambrose C.S.: In-office influenza vaccination by US pediatric providers varies greatly and is higher among smaller offices. Clin Pediatr (Phila) 2012; 51: 551-559.
10. Traynor K.: First quadrivalent flu vaccine approved. Am J Health Syst Pharm 2012; 69: 538.
11. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2012-2013 northern hemisphereinfluenza season. Weekly Epidemiological Record 2012; 87: 83-95.
12. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2013-14 northern hemisphere influenza season. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2013_14_north/en/.
13. U.S. Food and Drug Administration 2007. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. Available from: https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM091985.pdf.
14. European Medicines Agency 2016. Guideline on Influenza Vaccines. Non-clinical and Clinical Module. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211324.pdf.
15. Pépin S., Donazzolo Y., Jambrecina A., Salamand C., Saville M.: Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013; 31: 5572-5578.
16. Sesay S., Brzostek J., Meyer I. et al.: Safety, immunogenicity, and lot-to-lot consistency of a split-virion quadrivalent influenza vaccine in younger and older adults: a phase III randomized, double-blind clinical trial. Hum Vaccin Immunother. October 2017:0. doi:10.1080/21645515.2017.1384106.
17. Ustawa z dnia 12 maja 2011 r. o refundacji leków, środków spożywczych specjalnego przeznaczenia żywieniowego oraz wyrobów medycznych. (Dz.U. 2011 nr 122 poz. 696) Available from: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20111220696.
18. Chit A., Roiz J., Aballea S.: An Assessment of the Expected Cost-Effectiveness of Quadrivalent Influenza Vaccines in Ontario, Canada Using a Static Model. PLoS One. 2015; 10: e0133606.
19. EuroMOMO. European monitoring of excess mortality for public health action. Available from: www.euromomo.eu.
20. Schanzer D.L., Tam T.W., Langley J.M., Winchester B.T.: Influenza-attributable deaths, Canada 1990-1999. Epidemiol Infect 2007;135:1109-16.
21. Thompson W.W., Shay D.K., Weintraub E. et al.: Influenza associated hospitalizations in the United States. JAMA 2004;292:1333-40.
22. Ernst & Young 2013. Grypa i jej koszty. Wstępne studium w projekcie dotyczącym wypracowania rozwiązania na poziomie narodowym umożliwiającego istotne zwiększenie wyszczepialności przeciw grypie sezonowej w Polsce. Available from: http://opzg.cn-panel.pl/resources/dokumenty/dla-pracodawcow/Grypa-i-jej-koszty-w-Polsce.pdf.
23. Opinia Rady Konsultacyjnej w sprawie przygotowania analizy zasadności finansowania ze środków publicznych profilaktycznych szczepień przeciw grypie sezonowej jako szczepień obowiązkowych w wybranych grupach ryzyka dla produktów leczniczych Agrippal, Fluarix, Influvac, Vaxigrip/Vaxigrip Junior, Infleksal V. Available from: http://wwwold.aotm.gov.pl/assets/files/rada/opinie/opinia9.pdf.
24. Uhart M., Bricout H., Clay E., Largeron N.: Public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines compared to trivalent influenza vaccines in Europe. Hum Vaccin Immunother. 2016; 12: 2259-68.
25. Barbieri M., Patarnello F., Boccalini S. et al.: Quadrivalent versus trivalent influenza vaccine: Is it good value for money?. Value Health 2013; 16(7):A357.







_strona_04.png)